Summary
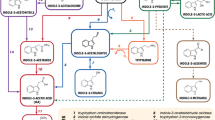
The combined activities of the Agrobacterium tumefaciens T-DNA genes 1 and 2 are sufficient to induce tumorous growth on several plants, by introducing a new auxin biosynthetic pathway in infected cells. We have isolated Nicotiana tabacum plants containing only gene 1 or gene 2. These plants, respectively called rG1 and rG2, grow and develop in a normal fashion, indicating that neither the gene 1 nor the gene 2 activity by itself interferes with the endogenous auxin metabolism in plants. Previous evidence indicated that the auxin biosynthetic pathway of Pseudomonas savastanoi and that proposed to be encoded by the T-DNA of Agrobacterium tumefaciens are similar. When rG2 plants were infected with non-oncogenic A. tumefaciens or Escherichia coli strains that harbour the P. savastanoi iaaM gene (responsible for indole-3-acetamide synthesis) root and callus formation at the infection site was readily observed. This shows that the product of iaaM, indole-3-acetamide, is an in vivo substrate for the gene 2 encoded enzyme and supports the proposal that the gene 1-encoded enzyme is involved in the synthesis of indole-3-acetamide in transformed plants. This result offers new insights in evolution of bacteria and plants involved in pathogenic and symbiotic interactions.
Similar content being viewed by others
Abbreviations
- IAM:
-
indole-3-acetamide
- IAA:
-
indole-3-acetic acid
References
Akiyoshi DE, Klee H, Amasino RM, Nester EW, Gordon MP (1984) T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proc Natl Acad Sci USA 81:5994–5998
Barry GF, Rogers SG, Fraley RT, Brand L (1984) Identification of a cloned cytokinin biosynthetic gene. Proc Natl Acad Sci USA 81:4776–4780
Birnboim HC, Doly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523
Bolivar F, Betlach M, Heyneker HL, Shine J, Rodriguez R, Boyer HW (1977) Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene 2:75–93
Braun AC (1956) The activation of a growth-substance system accompanying the conversion of normal to tumor cells in crown gall. Cancer Res 16:53–56
Colson C, Glover SW, Symonds N, Stacey KA (1965) The location of genes for host controlled modification and restriction in Escherichia coli K12. Genetics 52:1043–1050
Comai L, Kosuge T (1980) Involvement of plasmid deoxyribonucleic acid in indoleacetic acid synthesis in Pseudomonas savastanoi. J Bacterial 143:950–957
Comai L, Kosuge T (1982) Cloning and characterization of iaaM, a virulence determinant of Pseudomonas savastanoi. J Bacteriol 149:40–46
Deblaere R, Bytebier B, De Greve H, Deboeck F, Schell J, Van Montagu M, Leemens J (1985) Efficient octopine Ti plasmidderived vectors for Agrobacterium-mediated gene transfer to plants. Nucl Acids Res 13:4777–4788
De Cleene M, De Ley J (1976) The host range of crown-gall. Bot Rev 42:389–466
Depicker A, De Wilde M, De Vos G, De Vos R, Van Montagu M, Schell J (1980) Molecular cloning of overlapping segments of the nopaline Ti-plasmid pTiC58 as a means to restriction endonuclease mapping. Plasmid 3:193–211
De Vos G, De Beuckeleer M, Van Montagu M, Schell J (1981) Restriction endonuclease mapping of the octopine tumor inducting pTiAch5 of Agrobacterium tumefaciens. Plasmid 6:249–253
Dhaese P, De Greve H, Decraemer H, Schell J, Van Montagu M (1979) Rapid mapping of transposon insertion and deletion mutations in the large Ti-plasmids of Agrobacterium tumefaciens. Nucleic Acids Res 7:1837–1849
Engler G, Depicker A, Maenhaut R, Villarroel-Mandiola R, Van Montagu M, Schell J (1981) Physical mapping of DNA base sequence homologies between an octopine and a nopaline Ti-plasmid of Agrobacterium tumefaciens. J Mol Biol 152:183–208
Garfinkel DJ, Simpson RB, Ream LW, White FF, Gordon MP, Nester EW (1981) Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell 27:143–153
Gheysen G, Dhaese P, Van Montagu M, Schell J (1985) DNA flux across genetic barriers: the crown gall phenomenon. In: Hohn B, Dennis ES (eds) Genetic flux in plants (Advances in Plant Gene Research, vol 2). Springer Verlag, Wien, pp 11–47
Greene EM (1980) Cytokinin production by microorganisms. Bot Rev 46:25–73
Hamilton RH, Fall MZ (1971) The loss of tumor initiating ability in Agrobacterium tumefaciens by incubation at high temperature. Experientia 27:229–230
Herrera-Estrella L, De Block M, Messens E, Hernalsteens J-P, Van Montagu M, Schell J (1983) Chimeric genes as dominant selectable markers in plant cells. EMBO J 2:987–995
Holsters M, Silva B, Van Vliet F, Genetello C, De Block M, Dhaese P, Depicker A, Inzé D, Engler G, Villarroel R, Van Montagu M, Schell J (1980) The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid 3:212–230
Inzé D, Follin A, Van Lijsebettens M, Simoens C, Genetello C, Van Montagu M, Schell J (1984) Genetic analysis of the individual T-DNA genes of Agrobacterium tumefaciens; further evidence that two genes are involved in indole-3-acetic acid synthesis. Mol Gen Genet 194:265–274
Janssens A, Engler G, Zambryski P, Van Montagu M (1984) The nopaline C58 T-DNA region is transcribed in Agrobacterium tumefaciens. Mol Gen Genet 195:341–350
Joos H, Inzé D, Caplan A, Sormann M, Van Montagu M, Schell J (1983) Genetic analysis of T-DNA transcripts in nopaline crown galls. Cell 32:1057–1067
Kemper E, Waffenschmidt S, Weiler EW, Rausch T, Schröder J (1985) T-DNA encoded auxin formation in crown gall cells. Planta 163:257–263
Klapwijk PM, Van Beelen P, Schilperoort RA (1979) Isolation of a recombinant deficient Agrobacterium tumefaciens mutant. Mol Gen Genet 173:171–175
Kosuge T, Heskett MG, Wilson EE (1966) Microbial synthesis and degradation of indole-3-acetic acid. I. The conversion of L-tryptophan J Biol Chem 241:3738–3744
Leemans J, Shaw C, Deblaere R, De Greve H, Hermalsteens J-P, Maes M, Van Montagu M, Shell J (1981) Site-specific mutagenesis of Agrobacterium Ti plasmids and transfer of genes to plant cells. J Mol Appl Genet 1:149–164
Leemans J, Deblaere R, Willmitzer L, De Greve H, Hernalsteens J-P, Van Montagu M, Schell J (1982) Genetic identification of functions of TL-DNA transcripts in octopine crown galls. EMBO J 1:147–152
Linsmaier EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18:100–127
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning, a laboratory manual. Cold Spring Harbor Laboratory, New York
Márton L, Wullems GJ, Molendijk L, Schilperoort RA (1979) In vitro transformation of cultured cells from Nicotiana tabacum by Agrobacterium tumefaciens. Nature (London) 277:129–130
Nester EW, Gordon MP, Amasino RM, Yanofsky MF (1984) Crown gall: a molecular and physiological analysis. Ann Rev Plant Physiol 35:387–413
Ooms G, Hooykaas PJ, Moleman G, Schilperoort RA (1981) Crown gall plant tumors of abnormal morphology, induced by Agrobacterium tumefaciens carrying mutated octopine Ti plasmids; analysis of T-DNA functions. J Gene 14:33–50
Ooms G, Molendijk L, Schilperoort RA (1982) Double infection of tobacco plants by two complementing octopine T-region mutants of Agrobacterium tumefaciens. Plant Mol Biol 1:217–226
Petit A, Tempé J (1985) The function of T-DNA in nature. In: Van Vloten-Doting L, Groot GSP, Hall TC (eds) Molecular form and function of the plant genome. Plenum Press, New York, in press
Rao RN, Rogers SG (1979) Plasmid pKC7: a vector containing ten restriction endonuclease sites suitable for cloning DNA fragments. Gene 7:79–82
Schröder G, Klipp W, Hillebrand A, Ehring R, Koncz C, Schröder J (1983) The conserved part of the T-region in Ti-plasmids expresses four proteins in bacteria. EMBO J 2:403–409
Schröder G, Waffenschmidt S, Weiler EW, Schröder J (1984) The T-region of Ti plasmids codes for an enzyme synthesizing in-dole-3-acetic acid. Eur J Biochem 138:387–391
Sembdner G, Gross D, Liebisch H-W, Schneider G (1980) Molecular aspects of plant hormones. In: MacMillan J (ed), Hormonal regulation of development (Encyclopedia of Plant Physiology, new series, vol 9). Springer-Verlag, Berlin, pp 281–289
Skoog F, Miller CO (1957) Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol 11:118–131
Smidt M, Kosuge T (1978) The role of indole-3-acetic acid accumulation by alfa-methyl tryptophan resistant mutants of Pseudomonas savastanoi in gall formation on oleanders. Physiol Plant Pathol 13:203–214
Thomashow LS, Reeves S, Thomashow MF (1984) Crown gall oncogenesis: evidence that a T-DNA gene from the Agrobacterium Ti plasmid pTiA6 encodes an enzyme that catalyzes snythesis of indoleacetic acid. Proc Natl Acad Sci USA 81:5071–5075
Van Haute E, Joos H, Maes M, Warren G, Van Montagu M, Schell J (1983) Intergeneric transfer and exchange recombination of restriction fragments cloned in pBR322: a novel strategy for the reversed genetics of Ti plasmids of Agrobacterium tumefaciens. EMBO J 2:411–418
Van Larebeke N, Engler G, Holsters M, Van den Elsacker S, Zaenen I, Schilperoort RA, Schell J (1974) Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature (London) 252:169–170
Van Onckelen H, Rüdelsheim P, Inzé D, Follin A, Messens E, Horemans S, Schell J, Van Montagu M, De Greef J (1985) Tobacco plants transformed with the Agrobacterium T-DNA gene 1 contain high amounts of indole-3-acetamide. FEBS Lett 181:373–376
Willmitzer L, Dhaese P, Schreier PH, Schmalenbach W, Van Montagu M, Schell J (1983) Size, location, and polarity of T-DNA-encoded transcripts in nopaline crown gall tumors; evidence for common transcripts present in both octopine and nopaline tumors. Cell 32:1045–1056
Zambryski P, Joos H, Genetello C, Leemans J, Van Montagu M, Schell J (1983) Ti plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J 2:2143–2150
Author information
Authors and Affiliations
Additional information
Communicated by H. Saedler
Rights and permissions
About this article
Cite this article
Follin, A., Inzé, D., Budar, F. et al. Genetic evidence that the tryptophan 2-mono-oxygenase gene of Pseudomonas savastonoi is functionally equivalent to one of the T-DNA genes involved in plant tumour formation by Agrobacterium tumefaciens . Molec. Gen. Genet. 201, 178–185 (1985). https://doi.org/10.1007/BF00425657
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00425657