Summary
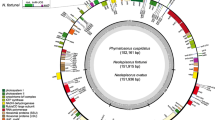
The evolution and recombination of chloroplast genome structure in the fern genus Osmunda were studied by comparative restriction site mapping and filter hybridization of chloroplast DNAs (cpDNAs) from three species — 0. cinnamomea, 0. claytoniana and 0. regalis. The three 144 kb circular genomes were found to be colinear in organization, indicating that no major inversions or transpositions had occurred during the approximately 70 million years since their radiation from a common ancestor. Although overall size and sequence arrangement are highly conserved in the three genomes, they differ by an extensive series of small deletions and insertions, ranging in size from 50 bp to 350 by and scattered more or less at random throughout the circular chromosomes. All three chloroplast genomes contain a large inverted repeat of approximately 10 kb in size. However, hybridizations using cloned fragments from the 0. cinnamomea and 0. regalis genomes revealed the absence of any dispersed repeats in at least 50% of the genome. Analysis with restriction enzymes that fail to cleave the 10 kb inverted repeat indicated that each of the three fern chloroplast genomes exists as an equimolar population of two isomeric circles differing only in the relative orientation of their two single copy regions. These two inversion isomers are inferred to result from high frequency intramolecular recombination between paired inverted repeat segments. In all aspects of their general organization, recombinational heterogeneity, and extent of structural rearrangement and length mutation, these fern chloroplast genomes resemble very closely the chloroplast genomes of most angiosperms.
Similar content being viewed by others
References
Aldrich J, Cherny B, Merlin E, Williams C, Mets L (1985) Curr Genet 9:233–238
Birnboim HC, Doly J (1979) Nucleic Acids Res 7:1513–1523
Bohnert HJ, Loffelhardt W (1982) FEBS Lett 150:403–406
Bohnert HJ, Crouse EJ, Schmitt JM (1982) Encycl Plant Physiol 14B:475–530
Bowman CM, Bonnard G, Dyer TA (1983) Theor Appl Genet 65:247–262
Coates D, Cullis CA (1982) Plant Mol Biol 1:183–189
Crouse EJ, Schmitt JM, Bohnert HJ (1985) Plant Mol Biol Rep 3:43–89
De Heij HT, Lustig H, Moeskops DJM, Bovenberg WA, Bisanz C, Groot GSP (1983) Cuff Genet 7:1–6
Fluhr R, Edelman M (1981) Nucleic Acids Res 9:6841–6853
Fluhr R, Fromm H, Edelman M (1983) Gene 25:271–280
Gelvin SB, Howell SH (1979) Mol Gen Genet 173:315–322
Gillham NW, Boynton JE, Harris EH (1985) In: Cavalier-Smith T (ed) DNA and evolution; natural selection and genome size. Wiley, New York, pp 299–351
Gordon KHJ, Crouse EJ, Bohnert HJ, Herrmann RG (1982) Theor Appl Genet 61:373–384
Herrmann RG, Palta HK, Kowallik (1980) Planta 148:319–327
Herrmann RG, Westhoff P, Alt J, Winter P, Tittgen J, Bisanz C, Sears BB, Nelson N, Hurt E, Hauska G, Viebrock A, Sebald W (1983) In: Ciferri 0, Dure L III (eds) Structure and function of plant genomes. Plenum Press, New York, pp 143–153
Ko K, Strauss NA, Williams JP (1983) Curr Genet 7:255–263
Lemieux B, Lemieux C (1985) Curr Genet 10:213–219
Lemieux C, Turmel M, Lee RW, Bellemare G (1985a) Plant Mol Biol 5:77–84
Lemieux C, Turmel M, Seligy VL, Lee RW (1985b) Curr Genet 9:139–145
Miller CN (1967) Contrib Mus Paleontol Univ Mich 21:139–203
Miller CN (1971) Contrib Mus Paleontol Univ Mich 23:105–169
Mubumbila M, Gordon KHJ, Crouse EJ, Burkard G, Weil JH (1983) Gene 21:257–266
Mubumbila M, Crouse EJ, Weil JH (1984) Curr Genet 8:379–385
Mubumbila M, Bowman CM, Droog F, Dyer T, Kuntz M, Weil JH (1985) Plant Mol Biol 4:315–320
Ohyama K, Yamano Y, Fukuzawa H, Komano T, Yamagishi H, Fujimoto S, Sugiura M (1983) Mol Gen Genet 189:1–9
Palmer JD (1982) Nucleic Acids Res 10:1593–1605
Palmer JD (1983) Nature (London) 301:92–93
Palmer JD (1985a) Annu Rev Genet 19:325–354
Palmer JD (1985b) In: MacIntyre RJ (ed) Monographs in evolutionary biology: molecular evolutionary genetics. Plenum Press, New York, pp 131–240
Palmer JD (1985c) Methods Enyzmol 118:167–186
Palmer JD, Stein DB (1982) Curr Genet 5:165–170
Palmer JD, Stein DB (1986) Curr Genet 10:823–833
Palmer JD, Thompson WF (1981) Proc Natl Acad Sci USA 78: 5533–5537
Palmer JD, Thompson WF (1982) Cell 29:537–550
Palmer JD, Shields CR, Cohen DB, Orton TJ (1983a) Theor Appl Genet 65:181–189
Palmer JD, Singh GP, Pillay DTN (1983b) Mol Gen Genet 190: 13–19
Palmer JD, Osorio B, Watson JC, Edwards H, Dodd J, Thompson WF (1984) In: Thornber JP, Staehelin LA, Hallick RB (eds) Biosynthesis of the photosynthetic apparatus: molecular biology, development and regulation. Liss, (UCLA Symposia on Molecular and Cellular Biology, New series, vol 14) New York, pp 273–283
Palmer JD, Jorgensen RA, Thompson WF (1985a) Genetics 109: 195–213
Palmer JD, Boynton JE, Gillham NW, Harris EH (1985b) In: Steinback KE, Bonitz S, Arntzen CJ, Bogorad L (eds) Molecular biology of the photosynthetic apparatus. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, pp 269–278
Rochaix JD (1978) J Mol Biol 126:597–617
Salts Y, Herrmann RG, Peleg N, Lavi U, Izhar S, Frankel R, Beckmann JS (1984) Theor Appl Genet 69:1–14
Spielmann A, Ortiz W, Stutz E (1983) Mol Gen Genet 190:5–12
Stein DB, Thompson WF, Belford HS (1979) J Mol Evol 13: 215–232
Takaiwa F, Sugiura M (1982) Nucleic Acids Res 10:2665–2676
Vieira J, Messing J (1982) Gene 19:257–268
Whitfeld PR, Bottomley W (1983) Annu Rev Plant Physiol 34: 279–310
Zurawski G, Clegg MT, Brown AHD (1984) Genetics 106:735–749
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stein, D.B., Palmer, J.D. & Thompson, W.F. Structural evolution and flip-flop recombination of chloroplast DNA in the fern genus Osmunda . Curr Genet 10, 835–841 (1986). https://doi.org/10.1007/BF00418530
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00418530