Abstract
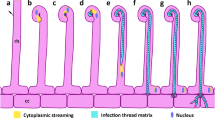
We have used spot-inoculation and new cytological procedures to observe the earliest events stimulated in alfalfa (Medicago sativa L.) roots by Rhizobium meliloti. Roots were inoculated with 1–10 nl of concentrated bacteria, fixed in paraformaldehyde, and after embedding and sectioning stained with a combination of acridine orange and DAPI (4′-6-diamidino-2-phenylindole hydrochloride). Normal R. meliloti provoke cell dedifferentiation and mitosis in the inner cortex of the root within 21–24 h after inoculation. This activation of root cells spreads progressively, leading to nodule formation. In contrast, the R. meliloti nodA and nodC mutants do not stimulate any activation or mitosis. Thus the primary and earliest effect of Rhizobium nod gene action is plant cellular activation. A rapid, whole-mount visualization by lactic acid shows that the pattern of nodule form varies widely. Some R. meliloti strains were found to be capable of stimulating on alfalfa roots both normal nodules and a “hybrid” structure intermediate between a nodule and a lateral root.
Similar content being viewed by others
References
Becking, J. (1975) Root nodules in non legumes. In: The development and function of roots, pp. 507–566, Torrey, J.G., Clarkson, D.T., eds. Academic Press, New York
Bhuvaneswari, T.V., Bhagwat, A.A., Bauer, W.D. (1981) Transient susceptibility of root cells in four common legumes to nodulation by Rhizobia. Plant Physiol. 68, 1144–1149
Calvert, H.E., Pence, M.K., Pierce, M., Malik, N.S.A., Bauer, W.D. (1984) Anatomical analysis of the development and distribution of Rhizobium infections in soybean roots. Can. J. Bot. 30, 2375–2384
Collins, J.M. (1983) Nodule initiation in white clover. B. Sci. thesis, Australian National University, Canberra, ACT, Australia
Dazzo, F.B., Hubbell, D.H. (1982) Control fo root hair infection. In: Ecology of nitrogen fixation, vol. 2, Rhizobium, pp. 275–309, Broughton, W., ed. Oxford University Press, Oxford, UK
Djordjevic, M.A., Schofield, P.R., Ridge, R.W., Morrison, N.A., Bassam, B.J., Plazinski, J., Watson, J.M., Rolfe, B.G. (1985) Rhizobium nodulation genes involved in root hair curling (Hac) are functionally conserved. Plant Mol. Biol. 4, 147–160
Finan, T.M., Hirsch, A.M., Leigh, J.A., Johansen, E., Kuldau, G.A., Deegan, S., Walker, G.C., Signer, E.R. (1985) Symbiotic mutants of Rhizobium meliloti that uncouple plant from bacterial differentiation. Cell 40, 869–877
Hirsch, A.M., Bang, M., Ausubel, F.M. (1983) Ultrastructural analysis of ineffective alfalfa nodules formed by nif::Tn5 mutants of Rhizobium meliloti. J. Bacteriol. 155, 367–380
Hirsch, A.M., Drake, D., Jacobs, T.W., Long, S.R. (1985) Nodules are induced on alfalfa roots by Agrobacterium tumefaciens and Rhizobium trifolii containing small segments of the Rhizobium meliloti nodulation region. J. Bacteriol. 161, 223–230
Hirsch, A.M., Long, S.R., Bang, M., Haskins, N., Ausubel, F.M. (1982) Structural studies of alfalfa roots infected with nodulation mutants of Rhizobium meliloti. J. Bacteriol. 151, 411–419
Hirsch, A.M., Wilson, K.J., Jones, J.D.G., Bang, M., Walker, V.V., Ausubel, F.M. (1984) Rhizobium meliloti nodulation genes allow Agrobacterium tumefaciens and Escherichia coli to form pseudonodules on alfalfa. J. Bacteriol. 158, 1133–1143
Jacobs, T.W., Egelhoff, T.T., Long, S.R. (1985) Physical and genetic map of a Rhizobium meliloti nodulation gene region and nucleotide sequence of nodC. J. Bacteriol. 162, 469–476
Libbenga, K.R., Harkes, P.A.A. (1973) Initial proliferation of cortical cells in the formation of root nodules in Pisum sativum L. Planta 114, 17–28
Meade, H.M., Long, S.R., Brown, S.E., Ruvkun, G.B., Ausubel, F.M. (1982) Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J. Bacteriol. 149, 114–122
Mulligan, J.T., Long, S.R. (1985) Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc. Natl. Acad. Sci. 82, 6609–6613
Murashige, T., Skoog, F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 474–497
Newcomb, W. (1976) A correlated light and electron microscopic study of symbiotic growth and differentiation in Pisum sativum root nodules. Can. J. Bot. 54, 2163–2186
Newcomb, W. (1981) Nodule morphogenesis and differentiation. In: Biology of the Rhizobiaceae, pp. 247–298, Giles, K.L., Atherly, A.G., eds. Academic Press, New York
Newcomb, W., Sippell, D., Peterson, R.L. (1979) The early morphogenesis of Glycine max and Pisum sativum root nodules. Can. J. Bot. 57, 2603–2616
Pierce, M., Bauer, W.D. (1983) A rapid regulatory response governing nodulation in soybean. Plant Physiol. 73, 286–290
Rossen, L., Johnston, A.W.B., Downie, J.A. (1984) DNA sequence of the Rhizobium leguminosarum nodulation genes nodAB and C required for root hair curling. Nucleic Acids Res. 12, 9497–9508
Sinnott, E.W. (1960) Plant morphogenesis. McGraw-Hill, New York
Trinick, M.J., Galbraith, J. (1977) Structure of root nodules formed by Rhizobium on the non-legume Trema cannabina (Parasponia). Arch. Microbiol. 108, 159–166
Truchet, G., Michel, M., Denarié, J. (1980) Sequential analysis of the organogenesis of lucerne (Medicago sativa) roots nodules using symbiotically-defective mutants of Rhizobium meliloti. Differentiation 16, 163–172
Turgeon, B.G., Bauer, W.D. (1982) Early events in the infection of soybean by Rhizobium japonicum. Time course and cytology of the initial infection process. Can. J. Bot. 60, 152–161
Turgeon, B.G., Bauer, W.D. (1985) Ultrastructure of infection-thread development during the infection of soybean by Rhizobium japonicum. Planta 163, 328–349
Turgeon, B.G., Bauer, W.D. (1986) Spot inoculation of soybean roots with Rhizobium japonicum. Protoplasma 115, 122–128
Vance, C.P., Johnson, L.E.B., Hardarson, G. (1980) Histological comparisons of plant and Rhizobium induced ineffective nodules in alfalfa. Physiol. Plant Pathol. 17, 167–173
Vasse, J.M., Truchet, G.L. (1984) The Rhizobium-legume symbiosis: Observation of root infection by bright-field microscopy after staining with methylene blue. Planta 161, 487–489
Yao, P.Y., Vincent, J.M. (1969) Host specificity in the root hair “curling factor” of Rhizobium spp. Aust. J. Biol. Sci. 22, 413–423
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dudley, M.E., Jacobs, T.W. & Long, S.R. Microscopic studies of cell divisions induced in alfalfa roots by Rhizobium meliloti . Planta 171, 289–301 (1987). https://doi.org/10.1007/BF00398674
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00398674