Summary
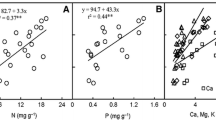
In the Chihuahuan Desert of southern New Mexico, both water and nitrogen limit the primary productivity of Larrea tridentata, a xerophytic evergreen shrub. Net photosynthesis was positively correlated to leaf N, but only in plants that received supplemental water. Nutrient-use efficiency, defined as photosynthetic carbon gain per unit N invested in leaf tissue, declined with increasing leaf N. However, water-use efficiency, defined as the ratio of photosynthesis to transpiration, increased with increasing leaf N, and thus these two measures of resource-use efficiency were inversely correlated. Resorption efficiency was not significantly altered over the nutrient gradient, nor was it affected by irrigation treatments. Leaf longevity decreased significantly with fertilization although the absolute magnitude of this decrease was fairly small, in part due to a large background of insect-induced mortality. Age-specific gas exchange measurements support the hypothesis that leaf aging represents a redistribution of resources, rather than actual deterioration or declining resource-use efficiency.
Similar content being viewed by others
References
Abul-Fatih HA, Bazzaz FA (1980) The biology of Ambrosia trifida L. IV. Demography of plants and leaves. New Phytol 84:107–111
Bamberg SA, Vollmer AT, Kleinkopf GE, Ackerman TL (1976) A comparison of seasonal primary production of Mojave desert shrubs during wet and dry years. Am Midl Nat 95:398–405
Bazzaz FA, Harper JL (1977) Demographic analysis of the growth of Linum usitatissimum. New Phytol 78:193–208
Bigger CM, Oechel WC (1982) Nutrient limitation of photosynthesis in arctic plants. Holarct Ecol. 5:158–163
Birk EM, Vitousek PM (1986) Nitrogen availability and nitrogen use efficiency in loblolly pine stands. Ecology 67:69–79
Bloom AJ, Chapin FS III, Mooney HA (1985) Resource limitation in plants-an economic analogy. Ann Rev Ecol Syst 16:363–392
Boerner REJ (1985) Foliar nutrient dynamics, growth, and nutrient use efficiency of Hamamelis virginiana in three forest microsites. Can J Bot 63:1476–1481
Bryant JP (1987) Feltleaf willow-snowshoe hare interactions: plant carbon/nutrient balance and floodplain succession. Ecology 68:1319–1327
Burk JH, Dick-Peddie WA (1973) Comparative production of Larrea divaricata Cav. on three geomorphic surfaces in southern New Mexico. Ecology 54:1094–1102
Chabot BF, Hicks DJ (1982) The ecology of leaf life spans. Ann Rev Ecol Syst 13:229–259
Chew RM, Chew AE (1965) The primary productivity of a desert shrub (Larrea tridentata) community. Ecol Mon 35:355–375
Chapin FS III, Kedrowski RA (1983) Seasonal changes in nitrogen and phosphorus fractions and autumn retranslocation in evergreen and deciduous taiga trees. Ecology 64:376–391
Chapin FS III, Bloom AJ, Field CB, Waring RH (1987) Plant responses to multiple environmental factors. BioScience 37:49–57
Chapin FS III, Johnson DA, McKendrick JD (1980) Seasonal nutrient allocation patterns in various tundra plant life forms in northern Alaska: implications for herbivory. J Ecol 68:189–209
Chazdon RL, Field CB (1987) Determinants of photosynthetic capacity in six rainforest Piper species. Oecologia 73:222–230
Coley PD, Bryant JP, Chapin FS III (1985) Resource availability and plant antiherbivore defense. Science 230:895–899
Comstock J, Ehleringer J (1986) Canopy dynamics and carbon gain in response to soil water availability in Encelia frutescens Gray, a drought-deciduous shrub. Oecologia 68:271–278
Cunningham GL, Syvertsen JP, Reynolds JF, Willson JM (1979) Some effects of soil moisture availability on above-ground production and reproductive allocation in Larrea tridentata (DC.) Cov. Oecologia 40:113–123
Damon RA Jr, Harvey WR (1987) Experimental design, ANOVA, and regression, Harper and Row, NY
Field C, Mooney HA (1983) Leaf age and seasonal effects on light, water, and nitrogen use efficiency in a California shrub. Oecologia 56:348–355
Field C, Mooney HA (1986) The photosynthesis-nitrogen relationship in wild plants. In: Givnish TJ (ed) On the economy of plant form and function. Cambridge University Press, Cambridge, pp 25–55
Field C, Merino J, Mooney HA (1983) Compromises between water-use efficiency and nitrogen-use efficiency in five species of California evergreens. Oecologia 60:384–389
Fife DN, Nambiar EKS (1984) Movement of nutrients in Radiata Pine needles in relation to the growth of shoots. Ann Bot 54:303–314
Fisher FM, Zak JC, Cunningham GL, Whitford WG (1988) Water and nitrogen effects on growth and allocation patterns of creosotebush in the northern Chihuahuan Desert. J Range Man 41:387–391
Gile LH, Hawley JW, Grossman RB (1981) Soils and geomorphology in the Basin and Range area of southern New Mexico-guidebook to the Desert Project. Memoir 39, New Mexico Bureau of Mines and Mineral Resources, Socorro, New Mexico, USA
Gulmon SL, Chu CC (1981) The effects of light and nitrogen on photosynthesis, leaf characteristics, and dry matter allocation in the chaparral shrub, Diplacus aurantiacus. Oecologia 49:207–212
Hadley EB, Bliss LC (1964) Energy relationships of alpine plants on Mt Washington, New Hampshire. Ecol Mon 34:331–357
Jonasson S, Chapin FS III (1985) Significance of sequential leaf development for nutrient balance of the cotton sedge, Eriophorum vaginatum L. Oecologia 67:511–518
Lajtha K (1987) The response to phosphorus fertilization and nutrient resorption efficiency in the desert shrub Larrea tridentata (DC.). Cov. Biogeochem 4:265–276
Lauenroth WK, Dodd JL, Sims PL (1978) The effects of water-and nitrogen-induced stresses on plant community structure in a semiarid grassland. Oecologia 36:211–222
Lennon JM, Aber JD, Melillo JM (1985) Primary production and nitrogen allocation of field grown sugar maples in relation to nitrogen availability. Biogeochem 1:135–154
Lightfoot DC, Whitford WG (1987) Variation in insect densities on desert creosotebush: is nitrogen a factor? Ecology 68:547–557
Miller HG, Cooper JM, Miller JD, Pauline OJL (1979) Nutrient cycles in pine and their adaptability to poor soils. Can J For Res 9:19–26
Monk CD (1966) An ecological significance of evergreeness. Ecology 47:504–505
Mooney HA, Rundel PW (1979) Nutrient relations of the evergreen shrub, Adenostoma fasciculatum, in the California chaparral. Bot Gaz 140:109–113
Mooney HA, Ferrar PJ, Slatyer RO (1978) Photosynthetic capacity and carbon allocation patterns in diverse growth forms of Eucalyptus. Oecologia 36:103–111
Natr L (1975) Influence of mineral nutrition on photosynthesis and the use of assimilates. In: Cooper JP (ed) Photosynthesis and productivity in different environments. Cambridge University Press, Cambridge, pp 537–556
Nelson DW, Sommers LE (1980) Total nitrogen analysis of soil and plant tissues. J Assoc Off Anal Chem 63:770–778
Nilsen ET (1986) Quantitative phenology and leaf survivorship of Rhododendron maximum in contrasting irradiance environments of the southern Appalachian Mountains. Amer J Bot 73:822–831
Nilsen ET, Muller WH (1981) Phenology of the drought-deciduous shrub Lotus scoparius: climatic controls and adaptive significance. Ecol Mon 51:323–341
Nilsen ET, Sharifi MR, Rundel PW, Virginia RA (1986) Influences of microclimatatic conditions and water relations on seasonal leaf dimorphism of Prosopis glandulosa, var. torreyana in the Sonoran Desert, California. Oecologia 69:95–100
Nilsen ET, Sharifi MR, Rundel PW (1987) Leaf dynamics in an evergreen and a deciduous species with even-aged leaf cohorts, from different environments. Amer Midl Nat 118:46–55
Oechel WC, Strain BR, Odening WR (1972) Tissue water potential, photosynthesis, 14C-labeled photosynthate utilization, and growth in the desert shurb Larrea divaricata Cav. Ecol Mon 42:127–141
Oechel WC, Lowell W, Jarrell W (1981) Nutrient and environmental controls on carbon flux in Mediterranean shrubs from California. In: Margaris NS, Mooney HA (eds) Components of productivity in Mediterranean climate regions—basic and applied aspects. Dr W Junk, The Hague, pp 51–60
Osman AM, Milthorpe FL (1971) Photosynthesis of wheat leaves in relation to age, illuminance and nutrient supply. II. results. Photosynthetica 5:61–70
Ostman NL, Weaver GT (1982) Autumnal nutrient transfer by retranslocation, leaching and litterfall in a chestnut oak forest in southern Illinois. Can J For Res 12:40–51
Pearcy RW, Ehleringer J (1984) Comparative ecophysiology of C3 and C4 plants. Plant Cell Environ 7:1–13
Reader RJ (1978) Contribution of overwintering leaves to the growth of three broad-leaved, evergreen shrubs belonging to the Ericaceae family. Can J Bot 56:1248–1261
Romney EM, Wallace A, Hunter RB (1978) Plant response to nitrogen fertilization in the northern Mohave Desert and its relationship to water manipulation. In: West NE, Skujins (eds) Nitrogen in desert ecosystems. US/IBP Synthesis Series. 9. Dowden, Hutchinson and Ross, Stroudsburg PA, USA pp 232–243
Runyon EH (1934) The organization of the creosote bush with respect to drought. Ecology 15:128–138
SAS Institute Inc (1982) SAS user's guide: statics, 1982 edition. SAS Institute Inc, Cary NC, USA
Schlesinger WH, Chabot BF (1977) The use of water and minerals by eyergreen and deciduous shrubs in Okefenokee Swamp. Bot Gaz 138:490–497
Seeman JR, Sharkey TD, Wang J, Osmond CB (1987) Environmental effects on photosynthesis, nitrogen-use efficiency, and metabolite pools in leaves of sun and shade plants. Plant Physiol 84:796–802
Sharifi MR, Nilsen ET, Virginia R, Rundel PW, Jarrell WM (1983) Phenological patterns of current season shoots of Prosopis glandulosa var. torreyana in the Sonoran Desert of southern California. Flora 173:265–277
Shaver GR (1981) Mineral nutrition and leaf longevity in an evergreen shrub, Ledum palustre ssp. decumbens. Oecologia 49:362–365
Shaver GR (1983) Mineral nutrition and leaf longevity in Ledum palustre: the role of individual nutrients and the timing of leaf mortality. Oecologia 56:160–165
Shaver GR, Melillo JM (1984) Nutrient budgets of marsh plants: efficiency concepts and relation to availability. Ecology 65:1491–1510
Shreve F (1942) The desert vegetation of North America. Bot Rev 8:195–246
Small E (1972) Photosynthetic rates in relation to nitrogen recycling as an adaptation to nutrient deficiency in peat bog plants. Can J Bot 50:2227–2233
Staaf H (1982) Plant nutrient changes in beech leaves during senescence as influenced by site characters. Acta Oecol/Oecol Plant 3:161–170
Stachurski A, Zimka JR (1975) Methods of studying ecosystems: leaf area, leaf production and withdrawal of nutrients from leaves of trees. Ekol Pol 23:637–648
Tolley-Henry L, Raper CD Jr (1986) Expansion and photosynthetic rate of leaves of soybean plants during onset of and recovery from nitrogen stress. Bot Gaz 147:400–406
Turner J (1977) Effect of nitrogen availability on nitrogen cycling in a Douglas-fir stand. Forest Sci 23:307–316
Turner J, Olsen PR (1976) Nitrogen relations in a Douglas-fir plantation. Ann Bot 40:1185–1193
Vitousek P (1982) Nutrient cycling and nutrient use efficiency. Am Nat 119:553–572
Vitousek PM (1984) Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology 65:285–298
Wall LL, Gehrke CW (1975) An automated total protein nitrogen method. J Assoc Off Anal Chem 58:1221–1226
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lajtha, K., Whitford, W.G. The effect of water and nitrogen amendments on photosynthesis, leaf demography, and resource-use efficiency in Larrea tridentata, a desert evergreen shrub. Oecologia 80, 341–348 (1989). https://doi.org/10.1007/BF00379035
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00379035