Summary
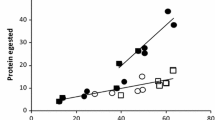
Black oak (Quercus velutina Lam.) and gray birch (Betula populifolia Marsh.) trees were defoliated in 0, 1, 2, or 3 successive years. Concentrations of 8 minerals, 4 sugars, and 25 amino acids in the foliage of these trees were measured when gypsy moth, Lymantria dispar (L.), reared on them were in instars I, III, IV, and V. These concentrations were tested for changes among years, and changes due to previous-and current-year defoliations. Most foliar constituents varied in concentration from year to year, though relatively few were affected by current or previous defoliations. In black oak, concentration of total free sugar measured during the fifth instar was reduced by current defoliation and correlated with gypsy moth pupal weight. In gray birch no decrease in sugar concentration due to defoliation was apparent, but pupal weights of gypsy moths reared on these trees were correlated with the ratio of total free sugar to calcium in the foliage measured during the fifth instar. Some implications of these apparent relations for gypsy moth larval growth and population dynamics are discussed.
Similar content being viewed by others
References
Arsenescu M, Ceianu I, Fratian B,Iliescu G, Popescu T, Simionescu A (1966) Iasi, depistara si prognaza inmultirii daunatorilor forestieri (Romanian). USDA Forest Service Translation, pp 5–67
Bess HA, Spurr SH, Littlefield EW (1947) Forest site conditions and the gypsy moth. Harvard Forest Bull No 22., p 56
Campbell RW, Sloan RJ (1978) Natural maintenance and decline of gypsy moth outbreaks. Environ Entomol 7:389–395
Chippendale GM, Reddy GPV (1974) Dietary carbohydrates: role in feeding behavior and growth of the southwestern corn borer, Diatraea grandiosella. J Insect Physiol 20:751–759
Cook JL, Housewaert MW, Kulman HM, Thompson LC (1978) Foliar mineral differences related to sawfly defoliation of white spruce. Environ Entomol 7:780–781
Dadd RH (1973) Insect nutrition: current developments and metabolic implications. Ann Rev Entomol 18:381–420
Dissescu G (1963) Research on the biology of the principal oak tree defoliating caterpillars. Report on doctoral thesis. Forestry department of the Brasov Polytechnical Institute. Brasov, Rumania, p 28
Feeny P (1970) Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51:565–581
Feeny P (1976) Plant apparency and chemical defence. In: J Wallace and R Mansell (eds) Biochemical interaction between plants and insects. Rec Adv Phytochem 10:1–40. Plenum Press, New York London
Fox LR, Macauley BJ (1977) Insect grazing on Eucalyptus in response to variation in leaf tannins and nitrogen. Oecologia (Berlin) 29:145–162
Furnival GM, Wilson RW (1974) Regressions by leaps and bounds. Technometrics 16:499–511
Gere G (1964) Change of weight, lipid and water content of Lymantria dispar L., with special regard to the chemical and energetic changes during insect metamorphosis and imaginal life. Acta Biol Hung 15:139–170
Green TR,Ryan CA (1972) Wound-induced proteinase inhibitor in plant leaves: a possible defense mechanism against insects. Science 175:776–777
Harvey GT (1974) Nutritional studies of eastern spruce budworm (Lepidoptera: Tortricidae). I. soluble sugars. Can Entomol 106:353–365
Haukioja E, Niemela P (1977) Retarded growth of a geometrid larva after mechanical damage to leaves of its host tree. Ann Zool Fennici 14:48–52
Hough JA, Pimentel D (1978) Influence of host foliage on development, survival and fecundity of the gypsy moth. Environ Entomol 7:97–102
Houston DR, Valentine HT (1977) Comparing and predicting forest stand susceptibility to gypsy moth. Can J Forest Res 7:447–461
Kovacevic Z (1956) Food-plant selection and the occurrence of plant pests (a contribution to the knowledge of population dynamics) (in German). Anz Schaedlingsled 29:97–101
Lovell PH, Oo HT, Sagar GR (1972) An investigation into the rate and control of assimilate movement from leaves in Pisum sativum. J Exp Bot 23:255–266
Mattson WJ (1980) Herbivory in relation to plant nitrogen content. Ann Rev Ecol Syst 11:119–161
Merker E (1960) The influence of tree condition on the mass increase of some forest pests. Z Angew Entomol 46:432–445
Mitchell HL (1936) Trends in the nitrogen, phosphorus, potassium and calcium content of the leaves of some forest trees during the growing season. Black Rock Forest Pap 1:30–44
Morrow PA, Fox LR (1980) Effects of variation in Eucalyptus essential oil yield on insect growth and grazing damage. Oecologia (Berlin) 45:209–219
Onuf CP, Teal JM, Valiela I (1977) Interactions of nutrients, plant growth and herbivory in a mangrove ecosystem. Ecology 58:514–516
Rausher MD (1981) Host plant selection by Battus philenor butterflies: the roles of predation, nutrition, and plant chemistry. Ecol Monogr 51:1–20
Rhoades DF, Cates RG (1976) Toward a general theory of plant antiherbivore chemistry. In: J Wallace and R Mansell (eds) Biological interaction between plants and insects. Rec Adv Phytochem 10:168–213. Plenum Press, New York London
Valentine HT, Houston DR, (1979) A discriminant function for identifying mixed-oak stand susceptibility to gypsy moth defoliation. Forest Sci 25:468–474
Valentine HT, Talerico RL (1980) Gypsy moth larval growth and consumption on red oak. Forest Sci 26:599–605
Wallner WE, Walton GS (1979) Host defoliation: a possible determinant of gypsy moth population quality. Ann Entomol Soc Am 72:62–67
White TCR (1974) A hypothesis to explain outbreaks of looper caterpillars, wih special reference to populations of Selidosema suavis in a plantation of Pinus radiata in New Zealand. Oecologia (Berlin) 16:279–301
White TCR (1978) The importance of a relative shortage of food in animal ecology. Oecologia (Berlin) 33:71–86
Zimmermann MH (1960) yLongitudinal and tangential movement within the sieve-tube system of white ash (Fraxinus americana L.). Beih Z Schweiz Forstv 30:289–300
Zimmermann MH, Brown CL (1974) Tree structure and function. Springer-Verlag, New York, Inc, p 336
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Valentine, H.T., Wallner, W.E. & Wargo, P.M. Nutritional changes in host foliage during and after defoliation, and their relation to the weight of gypsy moth pupae. Oecologia 57, 298–302 (1983). https://doi.org/10.1007/BF00377171
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00377171