Summary
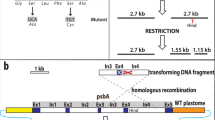
Photosynthetic mutants of the cyanobacterium Synechocystis PCC 6803 were produced by a random cartridge mutagenesis method leading to gene inactivation. This procedure relies on random ligation of an Escherichia coli kanamycin resistance (Kmr) gene to restriction fragments of genomic DNA from the host. Then recombination occurring during transformation promotes integration of the marker gene into the genome of the recipient cells. Several mutants impaired in photosynthesis were obtained by this procedure. All are partially or totally defective in photosystem II activity and some of them also harbour a functionally modified photosystem I. Restriction and recombination data showed that one mutant (AK1) is best explained as an insertion of the Kmr gene into an AvaII restriction site of the gene psbD-1. All other harbour a deletion, ranging from at least 1.15 kb (AK3) to more than 50 kb (AK9), which partly or fully overlaps the genes psbB and/or psbD-1, depending on the mutant. A genetic-physical map of the more than 60 kb region of the cyanobacterial genome harbouring the genes psbB, psbC and psbD-1 was constructed by combining published sequence data on these genes with the results of recombination and restriction mapping.
Similar content being viewed by others
References
Alt J, Morris J, Weshoff P, Herrman R (1984) Nucleotide sequence of the clustered genes for the 44 kd chlorophyll a apoprotein and the “32 kd”-like protein of the photosystem II reaction center in the Spinach plastid chromosome. Curr Genet 8:597–606
Astier C, Elmorjani K, Meyer I, Joset F, Herdman M (1984) Photosynthetic mutants of the cyanobacteria Synechocystis Sp strains PCC 6714 and PCC 6803: sodium p-hydroxymercuribenzoate as a selective agent. J Bacteriol 158:659–664
Berends T, Gamble P, Mullet J (1987) Characterization of the barley chloroplast transcription units containing psaA-psaB and psbD-psbC. Nucleic Acids Res 15:5217–5240
Bookjans G, Stummann B, Rasmussen O, Henningsen K (1986) Structure of a 32 kb region of pea chloroplast DNA containing the gene for the 44 kb photosystem II polypeptide. Plant Mol Biol 6:359–366
Bottin H, Mathis P (1985) Interaction of plastocyanin with the photosystem I reaction center: a kinetic study by flash absorption spectroscopy. Biochemistry 24:6453–6460
Briantais J-M, Vernotte C, Olive J, Wollman FA (1984) Kinetics of cation-induced changes in PSII fluorescence and of the lateral distribution of the two photosystems in the thylakoid membranes of pea. Biochim Biophys Acta 706:1–8
Briantais J-M, Vernotte C, Krause JH, Weis E (1986) Chlorophyll a fluorescence of higher plants: chloroplasts and leaves. In: Govindjee, Amesz J, Forks D (eds) Light emission by plants and bacteria. Academic Press, New York, pp 539–583
Buzby JS, Porter RD, Stevens S (1985) Expression of the E. coli lac Z gene on a plasmid vector in a cyanobacterium. Science 230:805–807
Castets A-M, Houmard J, Tandeau de Marsac N (1986) Is cell motility a plasmid-encoded function in the cyanobacterium Synechocystis 6803? FEMS Microbiol Lett 37:277–281
Chang ACY, Cohen SN (1978) Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the p 15 A cryptic miniplasmid. J Bacteriol 134:1141–1156
Chauvat F, de Vries L, van der Ende A, van Arkel GA (1986) A host-vector system for gene cloning in the cyanobacterium Synechocystis PCC 6803. Mol Gen Genet 204:185–191
Chauvat F, Labarre J, Ferino F (1988) Development of genes transfer systems for the cyanobacterium Synechocystis PCC 6803. Plant Physiol Biochem 26:629–637
Chisholm D, Williams JGK (1988) Nucleotide sequence of psbC, the gene encoding the CP-43 chlorophyll a — binding protein of photosystem II, in the cyanobacterium Synechocystis 6803. Plant Mol Biol 10:293–301
Debus RJ, Barry BA, Babcock GT, McIntosh L (1988) Site-directed mutagenesis identifies a tyrosine radical involved in the photosynthetic oxygen evolving system. Proc Natl Acad Sci USA 85:425–427
Demming B, Winter K, Krüger A, Czygan FC (1987) Photoinhibition and zeaxanthin formation in intact leaves: a possible role of the xanthophyll cycle in the dissipation of excess of light energy. Plant Physiol 84:218–224
Gray JC, Blyden ER, Eccles CJ, Dum PPJ, Hird SM, Hoglund AS, Kaethner TM, Smith AG, Willey DL, Dyer TA (1987) Chloroplast genes for photosynthetic membranes components. In: Biggins J, (ed) Progress in photosynthesis research, vol IV. Martinus Nijhoff, Dordrecht, pp 617–624
Grigorieva G, Shestakov S (1982) Transformation in the cyanobacterium Synechocystis Sp 6803. FEMS Microbiol Lett 13:367–370
Jansson C, Debus RJ, Osiewacz HD, Gurevitz M, McIntosh L (1987) Construction of an obligate photoheterotrophic mutant of the cyanobacterium Synechocystis 6803. Plant Physiol 85:1021–1025
Kuhlemeier CJ, Logtenberg T, Stoorvogel W, van Heughten HAA, Borrias WE, van Arkel GA (1984) Cloning of nitrate reductase genes from the cyanobacterium Anacystis nidulans. J Bacteriol 159:36–41
Kyle DJ (1987) The biochemical basis for photoinhibition of photosystem II. In: Kyle DJ, Osmond CB, Arntzen CJ (eds) Photoinhibition. Elsevier Science Publishers, Amsterdam, pp 197–226
Labarre J, Thuriaux P, Chauvat F (1987) Genetic analysis of amino acid transport in the facultatively heterotrophic cyanobacterium Synechocystis Sp Strain 6803. J Bacteriol 169:4668–4673
Manarelli BM, Lacks SA (1984) Ectopic integration of chromosomal genes in Streptococcus. J Bacteriol 160:867–873
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Morisson DA, Trombe M-C, Hayden MK, Waszak GA, Chen J-D (1984) Isolation of transformation-deficient Streptococcus pneumoniae mutants defective in control of competence, using insertion-duplication mutagenesis with the erythromycin resistance determinant of pAMB1. J Bacteriol 159:870–876
Niaudet B, Ehrlich SD (1981) Insertional mutagenesis: use in cloning of Bacillus subtilis genes. In: Polsinelli M, Mazza G (eds) Transformation. Costwold Press, Oxford, pp 201–209
Osiewacz HD, McIntosh L (1987) Nucleotide sequence of a member of the psbA multigene family from the unicellular Synechocystis 6803. Nucleic Acids Res 15:10585–10586
Pakrasi HB, Williams JGK, Arntzen CJ (1988) Targeted mutagenesis of the psbE and psbF genes block photosynthetic electron transport: evidence for a functional role of cytochrome b 559 in photosystem II. EMBO J 7:325–332
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Tandeau de Marsac N, Haumard J (1987) Advances in cyanobacterial molecular genetics. In: Fay P, van Baalen C (eds) The cyanobacteria, Elsevier, Amsterdam, pp 251–302
Tandeau de Marsac N, Borrias WE, Kuhlemeier CJ, Castets A-M, van Arkel GA, van den Hondel CAMJJ (1982) A new approach for molecular cloning in cyanobacteria: cloning of an Anacystis nidulans met gene using a Tn901-induced mutant. Gene 20:111–119
Vermaas WFJ, Williams JGK, Rutherford AW, Mathis P, Arntzen CJ (1986) Genetically engineered mutant of the cyanobacterium Synechocystis 6803 lacks the photosystem II chlorophyll-binding protein CP-47. Proc Natl Acad Sci USA 83:9474–9477
Vermaas WFJ, Williams JGK, Arntzen CJ (1987a) Sequencing and modification of psbB, the gene encoding the CP-47 protein of photosystem II in the cyanobacterium Synechocystis 6803. Plant Mol Biol 8:317–326
Vermaas WFJ, Williams JGK, Chisholm DA, Arntzen CJ (1987b) Site-directed mutagenesis in the photosystem II gene psbD encoding the D2 protein. In: Biggins J (ed) Progress in photosynthesis research, vol IV Martinus Nijhoff, Dordrecht, pp 805–808
Vermaas WFJ, Williams JGK, Arntzen CJ (1987c) Site-directed mutations of two histidine residues in the D2 protein inactivate and destabilize photosystem II in the cyanobacterium Synechocystis 6803. Z Naturforsch [C] 42:762–768
Williams JGK, Chisholm DA (1987) Nucleotide sequences of both psbD genes from the cyanobacterium Synechocystis 6803. In: Biggins J (ed) Progress in photosynthesis research, vol IV, Martinus Nijhoff, Dordrecht, pp 809–812
Youvan DC, Marrs BL (1984) Molecular genetics and the light reactions of photosynthesis. Cell 39:1–3
Author information
Authors and Affiliations
Additional information
Communicated by H. Böhme
Rights and permissions
About this article
Cite this article
Chauvat, F., Rouet, P., Bottin, H. et al. Mutagenesis by random cloning of an Escherichia coli kanamycin resistance gene into the genome of the cyanobacterium Synechocystis PCC 6803: Selection of mutants defective in photosynthesis. Mol Gen Genet 216, 51–59 (1989). https://doi.org/10.1007/BF00332230
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00332230