Summary
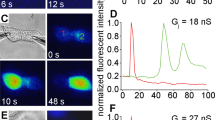
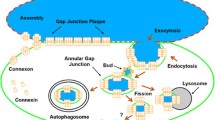
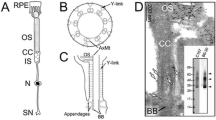
Transmission electron microscopy was used to examine internalized gap junctions (IGJ) in rabbit and rat ciliary epithelial cells. A prominent feature of all the specimens studied was the presence of different images of IGJ membrane that entrapped a portion of an adjoining cell. We documented and analyzed more than 500 gap junction (GJ) vacuoles and invaginations, the latter comprising less than 20% of all the structures examined. With ten exceptions found in non-pigmented cells, all the IGJ were unidirectionally internalized within the cytoplasm of pigmented epithelial cells. Morphological signs of autophagic degradation of GJ vacuoles were observed. An essential finding was that once a GJ membrane started to invaginate, a “lucidation” of a part of the protruding cytoplasm occurred; no planar GJ membranes exhibited such an alteration. The present findings suggest that IGJ derived from the epithelium of ciliary processes arise through an invagination-endocytosis mechanism and are degraded autophagically. This phenomenon may be relevant to aqueous humor production.
Similar content being viewed by others
References
Albertini DF, Fawcett DW, Olds PJ (1975) Morphological variations in gap junctions of ovarian granulosa cells. Tissue Cell 7:389–405
Amsterdam A, Rotmensch S, Furman A, Venter EA, Vlodavsky I (1989) Synergistic effect of human chorionic gonadotropin and extracellular matrix on in vitro differentiation of human granulosa cells: progesterone production and gap junction formation. Endocrinology 124:1956–1964
Brown D, Gluck S, Hartwig J (1987) Structure of the novel membrane-coating material in proton-secreting epithelial cells and indentification as an H+-ATPase. J Cell Biol 105:1637–1848
Dermietzel R, Völker M, Hwang T-K, Berzborn RJ, Meyer HE (1989) A 16 kDa protein co-isolating with gap junctions from brain tissue belonging to the class of proteolipids of the vacuolar H+-ATPases. FEBS Lett 253:1–5
Kogan M, Pappas GD (1975) Atypical gap junctions in the ciliary epithelium of albino rabbit eye. J Cell Biol 66:671–676
Larsen WJ (1977) Structural diversity of gap junctions. A review. Tissue Cell 9:373–394
Larsen WJ, Hai-Nan (1978) Origin and fate of cytoplasmic gap junctional vesicles in rabbit granulosa cells. Tissue Cell 10:585–598
Larsen WJ, Tung H-H, Murray SA, Swenson ChA (1979) Evidence for the participation of actin filaments and bristle coats in the internalization of gap junction membrane. J Cell Biol 83:576–587
Larsen WJ, Wert SE, Brunner GD (1987) Differential modulation of follicle cell gap junction populations at ovulation. Dev Biol 122:61–71
Merk FB, Albright JT, Botticelli ChR (1973) The fine structure of granulosa cell nexuses in rat ovarian follicles. Anat Rec 175:107–126
Parker JA, Goetzl EJ, Friedlaender MH (1986) Leukotrienes in the aqueous humor of patients with uveitis. Arch Ophthalmol 104:722–724
Peracchia C, Peracchia L (1980a) Gap junction dynamics: reversible effects of hydrogen ions. J Cell Biol 87:719–727
Peracchia C, Peracchia L (1980b) Gap junction dynamics: reversible effects of divalent cations. J Cell Biol 87:708–718
Raviola G, Raviola E (1978) Intercellular junctions in the ciliary epithelium. Invest Ophthalmol Vis Sci 17:958–981
Raviola E, Goodenough DA, Raviola G (1980) Structure of rapidly frozen gap junctions. J Cell Biol 87:273–279
Sasaki T, Garant PR (1986) Fate of annular gap junctions in the papillary cells of the enamel organ in the rat incisor. Cell Tissue Res 246:523–530
Sears ML (1985) Regulation of aqueous flow by the adenylate cyclase receptor complex in the ciliary epithelium. Am J Ophthalmol 100:194–198
Shibata Y, Izumi T, Yamamoto T (1989) Tissue-specific granularity of gap junction cytoplasmic surfaces revealed by rapid-freeze, deep-etch replicas. Anat Rec 223:113–120
Spray DC, Harris AL, Bennett MVL (1981) Gap junctional conductance is a simple and sensitive function of intracellular pH. Science 211:712–715
Spray DC, Fujita M, Saez JC, Choi H, Watanabe T, Hertzberg E, Rosenberg LC, Reid LM (1987) Proteoglycans and glycosaminoglycans induce gap junction synthesis and function in primary liver cultures. J Cell Biol 105:541–551
Tenkova T, Chaldakov GN (1988) Internalized gap junctions in rabbit ciliary body epithelium. A TEM study. Verh Anat Ges 82:961–962
Tenkova T, Kardalev S (1989) Nonpigmented epithelial cells in rabbit ciliary processes: ultrastructural features of proton-secreting cells. Ophthalmol 2:41–42 (in Bulgarian)
Tenkova T, Watanabe H, Chaldakov GN (1989) Gap junctions in rabbit ciliary process epithelium: a TEM and freeze-fracture study. 10th Natl Cong Anat Histol Embryol, 25–28 May, Stara Zagora (Bulgaria), 103A
Traub O, Look J, Paul D, Willecke K (1987) Cyclic adenosine monophosphate stimulates biosynthesis and phosphorylation of the 26 kDa gap junction protein in cultured mouse hepatocytes. Eur J Cell Biol 43:48–54
Vàzquez R, Carvajal JF, Pèrez E, Carretero J, Riesco JM, Blanco E (1988) Annular gap junctions in cuboidal ependymocytes of the median eminence of the rat after intraventricular administration of methysergide and tyrotropin releasing hormone. J Submicrosc Cytol Pathol 20:297–304
Watanabe H, Washioka H, Tonosaki A (1988) Gap junction and its cytoskeletal undercoats as involved in invagination-endocytosis. Tohoku J Exp Med 156:175–190
Wert SE, Larsen WJ (1989) Meiotic resumption and gap junction modulation in the cultured rat cumulus-oocyte complex. Gamete Res 22:143–162
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tenkova, T., Chaldakov, G.N. Internalized gap junctions in ciliary epithelium of rabbit and rat. Cell Tissue Res 261, 205–210 (1990). https://doi.org/10.1007/BF00329453
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00329453