Abstract
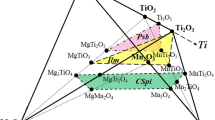
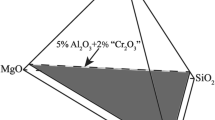
We have measured the mixing properties of Mn-Mg olivine and Mn-Mg garnet at 1300° C from a combination of interphase partitioning experiments involving these phases, Pt-Mn alloys and Mn-Mg oxide solid solutions. Activity coefficients of Mn dilute in Pt-Mn alloys at 1300° C/1 atm were measured by equilibrating the alloy with MnO at known f O 2. Assuming that the log f O 2 of the Mn-MnO equilibrium under these conditions is-17.80 (Robie et al. 1978), we obtain for γMn: logγMn = −5.25 + 3.67 XMn + 11.41X2 Mn Mixing properties of (Mn,Mg)O were determined by reversing the Mn contents of the alloys in equilibrium with oxide at known f O 2. Additional constraints were obtained by measuring the maximum extent of immiscibility in (Mn,Mg)O at 800 and 750° C. The data are adequately described by an asymmetric (Mn,Mg)O solution with the following upper and lower limits on nonideality: (a) WMn = 19.9kj/Mol; WMg = 13.7kj/Mol; (b) WMn = 19.9kj/Mol; WMg = 8.2kj/Mol; Olivine-oxide partitioning was tightly bracketed at 1300° C and oxide properties used to obtain activity-composition relations for Mn-Mg olivine. Despite strong M2 ordering of Mn in olivine, the macroscopic properties are adequately described by a symmetric model with: Wol = 5.5 ± 2.5 kj/mol (1-site basis) Using these values for olivine, garnet-olivine partitioning at 27 kbar/1300° C leads to an Mn-Mg interaction parameter in garnet given by: Wgt = 1.5 ± 2.5kJ/mol (1-site basis) Garnet-olivine partitioning at 9 kbar/1000° C is consistent with the same extent of garnet nonideality and the apparent absence of excess volume on the pyrope-spessartine join indicates that any pressure-dependence of WGt must be small. If olivine and garnet properties are both treated as unknown and the garnet-olivine partitioning data alone used to derive WOl and WGt, by multiple linear regression, best-fit values of 6.16 and 1.44 kJ/mol. are obtained. These are in excellent agreement with the values derived from metal-oxide, oxide-olivine and olivine-garnet equilibria.
Similar content being viewed by others
References
Akamatsu T, Fujino K, Kumazawa M, Fujimura A, Kato M, Sawamoto H, Yamanaka T (1988) Pressure and temperature dependence of cation distribution in Mg-Mn olivine. Phys Chem Miner 16:105–113
Berman RG (1990) Mixing properties of Ca-Mg-Fe-Mn garnets. Am Mineral 75:328–344
Bohlen SR, Wall VJ, Boettcher AL (1983) Experimental investigation and application of garnet granulite equilibria. Contrib Mineral Petrol 83:52–61
Deer WA, Howie RA, Zussman J (1966) An introduction to the rock-forming minerals. Longmans, London
Elphick SC, Ganguly J, Loomis TP (1985) Experimental determination of cation diffusivities in aluminosilicate garnets. I. Experimental methods and interdiffusion data. Contrib Mineral Petrol 90:36–44
Fetry JM, Spear FS (1978) Experimental calibration of partitioning of Fe and Mg between biotite and garnet. Contrib Mineral Petrol 66:113–117
Ganguly J, Kennedy GC (1974) The energetics of natural garnet solid solution. I. Mixing of the aluminosilicate end-members. Contrib Mineral Petrol 48:137–148
Ganguly J, Saxena SK (1984) Mixing properties of aluminosilicate garnets: constraints from natural and experimental data and applications to geothermobarometry. Am Mineral 69:88–97
Geiger CA, Newton RC, Kleppa OJ (1987) Enthalpy of mixing of synthetic almandine-grossular and almandine-pyrope garnets from high temperature solution calorimetry. Geochim Cosmochim Acta 51:1755–1763
Hackler RT, Wood BJ (1989) Experimental determination of Fe and Mg exchange between garnet and olivine and estimation of Fe-Mg mixing properties in garnet. Am Mineral 74:994–999
Hahn WC, Muan A (1970) Activities of the oxide components in NiO-MnO-MgO solid solutions. Mater Res Bull 5:955–964
Hardy HK (1953) A “subregular” solution model and its application to some binary alloy systems. Acta Metall 1:202–209
Hodges KV, Spear FS (1982) Geothermometry, geobarometry and the Al2SiO5 triple point at Mt. Moosilauke, New Hampshire. Am Mineral 67:1118–1134
Hultgren RP, Desai D, Hawkins DT, Gleiser M, Kelley KK (1973) Selected values of the thermodynamic properties of binary alloys. American Society for Metals, Metals Park, Ohio
Koziol AM (1990) Activity-composition relationships of binary Ca-Fe and Ca-Mn garnets determined by reversed displaced equilibrium experiments. Am Mineral 75:319–327
Lumpkin GR, Ribbe PH, Lumpkin NE (1983) Composition orderdisorder and lattice parameters of olivines: determinative methods for Mg-Mn and Mg-Ca silicate olivine. Am Mineral 68:1174–1182
Miyashiro A (1953) Calcium-poor garnet in relation to metamorphism. Geochim Cosmochim Acta 4:179–208
Newton RC, Perkins D III (1982) Thermodynamic calibration of geobarometers for charnockites and basic granulites based on the assemblages garnet-plagioclase-orthopyroxene (clinopyroxene)-quartz with applications to high grade metamorphism. Am Mineral 67:203–222
Novak GA, Colville AA (1989) A practical interactive least-squares cell-parameter program using an electronic spreadsheet and a personal computer. Am Mineral 74:488–490
O'Neill HStC (1988) Systems Fe-O and Cu-O: thermodynamic data for the equilibria Fe-“FeO”, Fe-Fe3O4, “FeO”-Fe3O4, Fe3O4-Fe2O3, Cu-Cu2O, and Cu2O-CuO from emf measurements. Am Mineral 73:470–486
O'Neill HStC, Wood BJ (1979) An experimental study of Fe-Mg partitioning between garnet and olivine and its calibration on a geothermometer. Contrib Mineral Petrol 70:59–70
O'Neill HStC, Pownceby MI, Wall VJ (1989) Activity-composition relations in FeTiO3-MnTiO3 ilmenite solid solutions from EMF measurements at 1050–1300 K. Contrib Mineral Petrol 103:216–222
Pownceby MI, Wall VJ, O'Neill HStC (1987) Fe-Mn partitioning between garnet and ilmenite: experimental calibration and applivations. Contrib Mineral Petrol 97:116–126
Robie RA, Hemmingway BS, Fisher JR (1978) Thermodynamic properties of minerals and related substances at 298.15K and 1 bar (105Pa) pressure and at higher temperature. US Geol Surv Bull 1452
Sack RO, Ghiorso MS (1989) Importance of considerations of mixing properties in establishing an internally consistent thermodynamic database: thermochemistry of minerals in the system Mg2SiO4-Fe2SiO4-SiO2. Contrib Mineral Petrol 102:41–68
Schwerdtfeger K, Muan A (1967) Equilibria in the system Fe-Mn-O involving “Fe,Mn)O” and (Fe,Mn)3O4 solid solutions. Trans Metall Soc AIME 239:1114–1119
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalogenides. Acta Crystallogr A32:751–767
Spear FS, Rumble D III (1986) Pressure, temperature and structural evolution of the Orfordville Belt, West-Central New Hampshire. J Petrol 27:1071–1093
Spear FS, Selverstone J (1983) Quantitative P-T paths from minerals: theory and tectonic applications. Contrib Mineral Petrol 83:348–357
Thompson JB Jr (1967) Thermodynamic properties of simple solutions. In: Abelson PH (ed) Researches in geochemistry, vol II. John Wiley and Sons, New York, pp 340–361
Vance D, O'Nions RK (1990) Isotope chronometry of zoned garnets: growth kinetics and metamorphic histories. Earth Planet Sci Letts 97:227–240
Wenk H-R, Raymond KN (1973) Four new structure refinements of olivine. Z Kristallogr 137:86–105
Wood BJ (1977) Experimental determination of the mixing properties of solid solutions with particular reference to garnet and clinopyroxene solutions. In: Fraser DG (ed) Thermodynamics in geology. D Reidel, Dordrecht, Netherlands, pp 11–27
Wood BJ, Kleppa OJ (1981) Thermochemistry of forsterite-fayalite olivine solutions. Geochim Cosmochim Acta 45:529–534
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wood, B.J., Hackler, R.T. & Dobson, D.P. Experimental determination of Mn-Mg mixing properties in garnet, olivine and oxide. Contr. Mineral. and Petrol. 115, 438–448 (1994). https://doi.org/10.1007/BF00320977
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00320977