Abstract
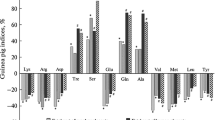
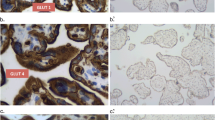
In the present study, the facilitative D-glucose transporter protein GLUT 1 was localised by immunohistochemistry in the placenta of human, marmoset (Callithrix jacchus) and rat at different developmental stages. A polyclonal antiserum agains a 13-amino-acid peptide of the GLUT 1 carboxy terminus was used. It identified a protein of around 50 kDa molecular weight in immunoblotting of the placental tissues. GLUT 1 was located in the syncytiotrophoblast, in cytotrophoblast cells and in fetal endothelium. Similar staining patterns, except in human extravillous cytotrophoblast cells, were observed at all differentiation stages, despite differences in the internal placental architecture of the species. In the marmoset placenta, GLUT 1 was undetectable in endothelial cells of maternal vessels. In rat placentae, trophoblastic giant cells, epithelial cells of both visceral and parietal yolk sac, yolk sac vessels and the stratum spongiosum were stained. Reichert's membrane did not immunoreact. Preadsorption of the antiserum with a 13-amino-acid peptide resulted in the loss of immunoreactivity. The results suggest that GLUT 1 is a prominent isoform of glucose transporters in mammalian placentae. It is generally abundant in placental cell populations bordering on the maternal and fetal circulations and may therefore facilitate an effective glucose supply to the fetus and placenta.
Similar content being viewed by others
References
Baly DL, Horuk R (1988) The biology and biochemistry of the glucose transporter. Biochim Biophys Acta 947:571–590
Beckman DA, Brent RL, Lloyd JB (1994) Pinocytosis in the rat visceral yolk sac: potential role in amino acid nutrition during the fetal period. Placenta 15:171–176
Bell GI, Kayano T, Buse JB, Burant CF, Takeda J, Lin D, Fukumoto H, Seino S (1990) Molecular biology of mammalian glucose transporters. Diabetes Care 13:198–208
Benirschke K, Kaufmann P (1990) The pathology of the human placenta, Springer, Berlin Heidelberg New York
Birnbaum MJ, Haspel HC, Rosen OM (1986) Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci USA 83:5784–5788
Bremer D (1983) Entwicklung und Charakterisierung der Plazenta von Callithrix jacchus. Thesis, Freie Universität Berlin
Cantle SJ, Kaufmann P, Luckhardt M, Schweikhart G (1987) Interpretation of syncytial sprouts and bridges in the human placenta. Placenta 8:221–234
Carstensen M, Leichtweiss H-P, Molsen G, Schröder H (1977) Evidence for a specific transport of D-hexoses across the human term placenta in vitro. Arch Gynäkol 222:187–196
Chinard FP, Danesino V, Hartmann WL, Huggett ASG, Paul W, Reynolds SRM (1956) The transmission of hexoses across the placenta in the human and the rhesus monkey. J Physiol (Lond) 132:289–303
Desoye G, Hartmann M, Blaschitz A, Dohr G, Hahn T, Kohnen G, Kaufmann P (1994) Insulin receptors in syncytiotrophoblast and fetal endothelium of human placenta. Immunohistochemical evidence for developmental changes in distribution pattern. Histochemistry 101:277–285
Doyle D, Baumann H, England B, Friedmann E, Hou E, Tweto J (1978) Brogenesis of plasma membrane glycoproteins in hepatoma tissue culture cells. J Biol Chem 253:965–973
Enders AC (1965) A comparative study of the fine structure of the trophoblast in several hemochorial placentas. Am J Anat 116:29–68
Englund AK, Lundahl P (1993) Isoelectric focusing in immobilized pH gradients of the glucose transporter from human red blood cell membranes. Electrophoresis 14:1307–1311
Franke M (1969) Feinstruktur der Plazenta. Elektronenmikroskopische Untersuchungen über die reifende und reife Plazenta der Ratte. Fischer, Jena
Gorga FR, Baldwin SA, Lienhard GE (1979) The monosaccharide transporter from human erythrocytes is heterogeneously glycosylated. Biochem Biophys Res Commun 91:955–961
Gossrau R, Merker H-J (1984) Ultrastructure and enzyme histochemistry of maternal blood vessels in the marmoset placenta. Histochemical J 16:395–398
Gould GW, Bell GI (1990) Facilitative glucose transporters: an expanding family. Trends Biochem Sci 15:18–23
Hahn T, Blaschitz A, Hartmann M, Lang I, Skofitsch G, Dohr G, Desoye G. (1994) Non-Michaelis-Menten kinetics of zerotrans glucose uptake by trophoblast cells from human term placentae and by choriocarcinoma (JEG-3/JAR) cells. Biol Chem Hoppe-Seyler 375:543–550
Haspel HC, Rosenfeld MG, Rosen OM (1988) Characterization of antisera to a synthetic carboxyl-terminal peptide of the glucose transporter protein. J Biol Chem 263:398–403
Heger HW, Neubert D (1983) Timing of ovulation and implantation in the common marmoset, Callithrix jacchus, by monitoring of estrogen and 6-β-hydroxy-pregnenolon in urine. Arch Toxicol 54:41–52
Herrera E, Palacin M, Martin A, Lasuncion MA (1985) Relationship between maternal and fetal fuels and placental glucose transfer in rats with maternal diabetes of varying severity. Diabetes 34 [Suppl] 2:42–46
Hill J (1932) On the developmental history of the primates. Philos Trans R Soc Lond [Biol] 221:45–178
Jansson T, Wennergren M, Illsley NP (1993) Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metabol 77:1554–1562
Jollie WP (1990) Development, morphology and function of the yolk sac placenta of laboratory rodents. Teratology 41:361–381
Koszalka TR, Andrew CL, Lloyd JB, Brent RL (1988) Carrier-mediated uptake of hexoses by the rat visceral yolk sac. Placenta 9:547–558
Leichtweiss H-P (1981) Carrier-mediated placental transfer. Placenta [Suppl] 1:115–124
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mueckler M (1990) Family of glucose-transporter genes. Implications for glucose homeostasis and diabetes. Diabetes 39:6–11
Ramsey EM (1982) The placenta. Human and animal. Praeger, New York
Reiber W, Malek A, Aegerter E, Sager R, Schneider H (1991) Bidirectional human placental glucose transfer in vitro prefers maternofetal direction. Placenta 12:430
Schneider H, Challier JC, Dancis J (1981) Transfer and metabolism of glucose and lactate in the human placenta perfused in vitro. Br J Obstet Gynecol 86:299–306
Schröder H, Schoch C, Elwers W, Leichtweiss H-P (1991) The artificially perfused guinea-pig yolk sac placenta: transfer and uptake of water, glucose and amino acids. Placenta 12:495–509
Stein WD (1967) The movement of molecules across cell membranes. Academic Press, New York
Takao Y, Akazawa S, Matsumoto K, Takino H, Akazawa M, Trocino RA, Maeda Y, Okuno S, Kawasaki E, Uotani S, Yokota A, Nagataki S (1993) Glucose transporter gene expression in rat conceptus during high glucose culture. Diabetologia 36:696–706
Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H (1992) Localization of erythrocyte/HepG2-type glucose transporter (GLUT1) in human placental villi. Cell Tissue Res 267:407–412
Thomas CR, Eriksson GL, Eriksson UJ (1990) Effects of maternal diabetes on placental transfer of glucose in rats. Diabetes 39:276–282
Waddell ID, Scott HM, Grant A, Burchell A (1991) Identification and characterization of a hepatic microsomal glucose transport protein. Biochem J 275:363–367
Wessling M, Pilch PF (1984) Characterization and solubilization of the cytochalasin B binding component from human placental microsomes. Biochem Biophys Acta 777:123–132
Wislocki G, Streeter G (1938) On the placentation of the macaque (Macaca mulatta) from time of implantation until the formation of the definitive placenta. Carnegie Contrib Embryol 27:1–65
Wolf HJ, Desoye G (1993) Immunohistochemical localization of glucose transporters and insulin receptors in human fetal membranes at term. Histochemistry 100:379–385
Zhou J, Bondy CA (1993) Placental glucose transporter gene expression and metabolism in the rat. J Clin Invest 91:845–852
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hahn, T., Hartmann, M., Blaschitz, A. et al. Localisation of the high affinity factilitative glucose transporter protein GLUT 1 in the placenta of human, marmoset monkey (Callithrix jacchus) and rat at different developmental stages. Cell Tissue Res 280, 49–57 (1995). https://doi.org/10.1007/BF00304510
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00304510