Summary
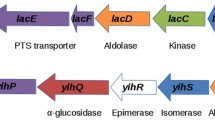
Four genes, nagR, A, B and E, clustered in the nag locus of Escherichia coli K12 and Klebsiella pneumoniae, were cloned and physically mapped, and the corresponding gene products involved in amino sugar metabolism identified. Expression of the nag genes was also analysed using a series of lacZ fusions. In both bacteria, the genes are arranged in two divergent operons and controlled by a common NagR repressor. The corresponding gene nagR was found to map in the first operon together with the promoter proximal gene nagB, encoding the enzyme d-glucosamine isomerase (deaminase) (NagB) and the middle gene nagA, coding for N-acetyl-glucosamine deacetylase (NagA). Polar mutations in nagB and nagA prevent the efficient expression of nagR and cause constitutive expression of all nag genes. This includes the gene nagE encoding Enzyme IINag of the phosphoenolpyruvate-dependent carbohydrate phosphotransferase system (PTS), encoded in the second divergently transcribed operon. No further gene is found in this operon which in both organisms is directly adjacent to the gene glnS. It is interesting that the NagR repressor also affects the mannose PTS (genes manX, Y, Z), the second transport system involved in amino sugar uptake and phosphorylation.
Similar content being viewed by others
References
Altenbuchner J, Schmid K, Schmitt R (1983) Tn1721-encoded tetracycline resistance: mapping of structural and regulatory genes mediating resistance. J Bacteriol 153:116–123
Bachmann BJ (1987) Linkage map of Escherichia coli K12. In: Neidhardt FC, Ingraham JL, Brooks Low K, Magasanik B, Schaechter M, Umbarger HE (eds) Escherichia coli and Salmonella typhimurium. ASM, Washington DC
Bates CJ, Pasternak CA (1965) Further studies on the regulation of amino sugar metabolism. Biochem J 96:147–154
Bremer E, Silhavy TJ, Weisemann JM, Weinstock GM (1984) λplacMu: a transposable derivative of bacteriophage lambda for creating lacZ protein fusions in a single step. J Bacteriol 158:1084–1093
Bremer E, Silhavy TJ, Weinstock GM (1985) Transposable λplacMu bacteriophages for creating lacZ operon fusions and kanamycin resistance insertions in Eschericha coli. J Bacteriol 162:1092–1099
Comb DG, Roseman S (1958) Glucosamine-6-phosphate deaminase. Biochim Biophys Acta 21:193–194
Ebner R, Lengeler JW (1988) Sucrose-specific Enzyme IISer of the bacterial phosphotransferase system: nucleotide sequence of the gene scrA from pUR400. Mol Microbiol 2:9–17
Erni B, Zanolari B (1986) The mannose permease of Escherichia coli consists of three proteins: Amino acid sequence and function in sugar transport, sugar phosphorylation, and penetration of phage DNA. J Biol Chem 262:5238–5247
Felton J, Michaelis S, Wright A (1980) Mutations in two unlinked genes are required to produce asparagine auxotrophy in Escherichia coli. J Bacteriol 142:221–228
Holmes RP, Russel RRB (1972) Mutations affecting amino sugar metabolism in E. coli K12. J Bacteriol 111:290–291
Imada A, Nozaki Y, Kawashima F, Yonida M (1977) Regulation of glucosamine utilization in Staphylococcus aureus and Escherichia coli. J Gen Microbiol 100:329–337
Jones-Mortimer MC, Kornberg HL (1980) Amino-sugar transport systems of Escherichia coli K12. J Gen Microbiol 117:369–376
Lengeler J (1975) Mutations affecting transport of the hexitols d-mannitol, d-glucitol, and galactitol in Escherichia coli K-12: isolation and mapping. J Bacteriol 124:26–38
Lengeler J (1979) Streptozotocin, an antibiotic superior to penicillin in the selection of rare bacterial mutations. FEMS Microbiol Lett 5:417–419
Lengeler JW (1980) Characterisation of mutants of Escherichia coli K12, selected by resistance to Streptozotocin. Mol Gen Genet 179:49–54
Lengeler J, Lin ECC (1972) Reversal of the mannitol-sorbitol diauxie in Escherichia coli. J Bacteriol 112:840–848
Lengeler J, Steinberger H (1978) Analysis of the regulatory mechanisms controlling the synthesis of the hexitol transport systems in Escherichia coli K-12. Mol Gen Genet 164:163–169
Levy GA, McAllan A (1959) The N-acetylation and estimation of hexosamines. Biochem J 73:127–132
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning. A laboratory manual. Cold Spring Harbour Laboratory, New York
Murray NE, Brammar WJ, Murray K (1977) Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet 150:53–61
Pardee AB, Prestidge LS (1961) The initial kinetics of enzyme induction. Biochim Biophys Acta 100:591–602
Peri KG, Waygood EB (1988) Sequence of cloned Enzyme IIN-acetyl-glucosamne of the phosphoenolpyruvate:N-acetylglucosamine phosphotransferase system of Escherichia coli. Biochemistry 27:6054–6061
Plumbridge J (1987) Organisation of the Escherichia coli chromosome between genes glnS and glnU, V. Mol Gen Genet 209:618–620
Postma PW, Lengeler JW (1985) Phosphoenolpyruvate: carbohydrate phosphotransferase system of bacteria. Microbiol Rev 49:232–269
Rogers JR, Ohgi T, Plumbridge J, Söll D (1988) Nucleotide sequences of the Escherichia coli nagE and nagB genes: the structural genes for the N-acetylglucosamine transport protein of the bacterial phosphoenolpyruvate: sugar phosphotransferase system and for glucosamine-6-phosphate deaminase. Gene 62:197–207
Saris PEJ, Palva ET (1987) The ptsL/pell/ptsM (manXYZ) locus consists of three genes involved in mannose uptake in Escherichia coli K-12. FEMS Microbiol Lett 44:371–376
Sarvas M (1971) Mutant of Escherichia coli K-12 defective in d-glucosamine biosynthesis. J Bacteriol 105:467–471
Shurvinton CE, Lloyd RG, Benson FE, Attfield PV (1984) Genetic analysis and molecular cloning of the Escherichia coli ruv gene. Mol Gen Genet 194:322–329
Sprenger G, Vogler A, Lengeler JW (1986) Selection of auxotrophic and carbohydrate-negative mutants in penicillin-resistant Klebsiella pneumoniae by nalidixic acid treatment. FEMS Microbiol Lett 37:299–304
Vogler A (1988) Molekulare Untersuchungen zum N-Acetylglucosamin-Stoffwechsel in Enterobakterien. Evolution des Transportsystems und seine Beteiligung an der Chemotaxis. Dissertation Universität Osnabrück
Vogler AP, Lengeler JW (1988) Complementation of a truncated membrane-bound Enzyme IINag from Klebsiella pneumoniae with a soluble Enzyme III in Escherichia coli K12. Mol Gen Genet 213:175–178
White RJ (1968) Control of amino sugar metabolism in Escherichia coli and isolation of mutants unable to degrade amino sugars. Biochem J 106:847–858
White RJ (1970) The role of the phosphoenolpyruvate phosphotransferase system in the transport of N-acetyl-glucosamine by Escherichia coli. Biochem J 118:89–92
Wu HC, Wu TC (1971) Isolation and characterization of a glucosamine-requiring mutant of Escherichia coli K-12 defective in glucosamine-6-phosphate synthetase. J Bacteriol 105:455–466
Author information
Authors and Affiliations
Additional information
Communicated by H. Hennecke
Rights and permissions
About this article
Cite this article
Vogler, A.P., Lengeler, J.W. Analysis of the nag regulon from Escberichia coli K12 and Klebsiella pneumoniae and of its regulation. Molec. Gen. Genet. 219, 97–105 (1989). https://doi.org/10.1007/BF00261163
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00261163