Abstract
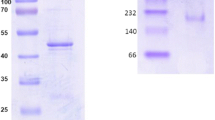
Anaerobically prepared cell extracts of Clostridium kluyveri grown on succinate plus ethanol contained high amounts of 4-hydroxybutyryl-CoA dehydratase, which catalyzes the reversible dehydration of 4-hydroxybutyryl-CoA to crotonyl-CoA. The enzyme was purified 12-fold under strictly anaerobic conditions to over 95% homogeneity and had a specific activity of 123 nkat mg-1. The finding of this dehydratase means that all of the enzymes necessary for fermentation of succinate plus ethanol by C. kluyveri have now been demonstrated to exist in this organism and confirms the proposed pathway involving a reduction of succinate via 4-hydroxybutyrate to butyrate. Interestingly, the enzyme is almost identical to the previously isolated 4-hydroxybutyryl-CoA dehydratase from Clostridium aminobutyricum. The dehydratase was revealed as being a homotetramer (m=59 kDa/subunit), containing 2±0.2 mol FAD, 13.6±0.8 mol Fe and 10.8±1.2 mol inorganic sulfur. The enzyme was irreversibly inactivated after exposure to air. Reduction by sodium dithionite also yielded an inactive enzyme which could be reactivated, however, up to 84% by oxidation with potassium hexacyanoferrate(III). The enzyme possesses an intrinsic vinylacetyl-CoA isomerase activity which was also found in 4-hydroxybutyryl-CoA dehydratase from C. aminobutyricum. Moreover, the N-terminal sequences of the dehydratases from both organisms were found to be 63% identical.
Similar content being viewed by others
References
Bader J, Günther H, Schleicher E, Simon H, Pohl S, Mannheim W (1980) Utilization of (E)-2-butenoate (crotonate) by Clostridium kluyveri and some other Clostridium species. Arch Microbiol 125: 159–165.
Bartsch RG, Barker HA (1961) A vinylacetyl-CoA isomerase from Clostridium kluyveri. Arch Biochem Biophys 92: 122–132.
Bergmeyer HU, Holz G, Klotzsch H, Lang G (1963) Phosphotrans-acetylase aus Clostridium kluyveri. Biochem Z 338: 114–121.
Bornstein BT, Barker HA (1948) The energy metabolism of Clostridium kluyveri and the synthesis of fatty acids. J Biol Chem 172: 659–669.
Buckel W (1992) Unusual dehydrations in anaerobic bacteria. FEMS Microbiol Rev 88: 211–232.
Cline JD (1969) Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr 14: 454–458.
Fish WW (1988) Rapid colorimetric micromethod for the quantitation of complexed iron in biological samples. Methods Enzymol 158: 357–364.
Härtel U, Eckel E, Koch J, Fuchs G, Linder D, Buckel W (1993) Purification of glutaryl-CoA dehydrogenase from Pseudomonas sp., an enzyme involved in the anaerobic degradation of benzoate. Arch Microbiol 159: 174–181.
Hofmeister AEM, Berger S, Buckel W (1992) The iron-sulfur-cluster-containing l-serine dehydratase from Peptostreptococcus asaccharolyticus. Stereochemistry of the deamination of l-threonine. Eur J Biochem 205: 743–749.
Hofmeister AEM, Buckel W (1992) (R)-Lactyl-CoA dehydratase from Clostridium propionicum. Stereochemistry of the dehydration of (R)-2-hydroxybutyryl-CoA to crotonyl-CoA. Eur J Biochem 206: 547–552.
Kenealy WR, Zeikus JG (1981) Influence of corrinoid antagonists on methanogen metabolism. J Bacteriol 146: 133–140.
Kenealy WR, Waselefsky DM (1985) Studies on the substrate range of Clostridium kluyveri; the use of propanol and succinate. Arch Microbiol 141: 187–194.
Klees AG, Linder D, Buckel W (1992) 2-Hydroxyglutaryl-CoA dehydratase from Fusobacterium nucleatum (subsp. nucleatum): an iron-sulfur flavoprotein. Arch Microbiol 158: 294–301.
Kuchta RD, Abeles RH (1985) Lactate reduction in Clostridium propionicum. Purification and properties of lactyl-CoA dehydratase. J Biol Chem 260: 13181–13189.
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Michel C, Buckel W, Linder D (1991) Purification of the coenzyme B12-containing 2-methyleneglutarate mutase from Clostridium barkeri by high performance liquid chromatography. J Chromatogr 587: 93–99.
Ramjee MN, Coggins JR, Hawkes TR, Lowe DJ, Thorneley RNF (1991) Spectrophotometric detection of a modified flavin mononucleotide (FMN) intermediate formed during the catalytic cycle of chorismate synthase. J Am Chem Soc 113: 8566–8567.
Ramjee MN, Balasubramanian S, Abell C, Coggins JR, Davies GM, Hawkes TR, Lowe DJ, Thorneley RNF (1992) Reaction of (6R)-6-F-EPSP with recombinant Escherichia coli chorismate synthase generates a stable flavin mononucleotide semiquinone radical. J Am Chem Soc 114: 3151–3153.
Scherf U, Buckel W (1991) Purification and properties of 4-hydroxybutyrate Coenzyme A transferase from Clostridium aminobutyricum. Appl Environ Microbiol 57: 2699–2702.
Scherf U, Buckel W (1993) Purification and properties of an iron-sulfur and FAD-containing 4-hydroxybutyryl-CoA dehydratase/vinylacetyl-CoA 239–3 from Clostridium aminobutyricum. Eur J Biochem 215: 421–429.
Schleicher E, Simon H (1976) On the mechanism and some properties of vinylacetyl-CoA Δ-isomerase of Clostridium kluyveri. Hoppe-Seyler's Z Physiol Chem 357: 535–541.
Schoberth S, Gottschalk G (1969) Consideration of the energy metabolism of Clostridium kluyveri. Arch Microbiol 65: 318–328.
Schweiger G, Dutscho R, Buckel W (1987) Purification of 2-hydroxyglutaryl-CoA dehydratase from Acidaminococcus fermentans, an iron-sulfur protein. Eur J Biochem 169: 441–449.
Schwörer B, Thauer RK (1991) Activities of formylmethanofuran dehydrogenase, methylentetrahydromethanopterin dehydrogenase, methylentetrahydromethanopterin reductase, and heterodisulfide reductase in methanogenic bacteria. Arch Microbiol 155: 459–465.
Simon EJ, Shemin D (1953) The preparation of S-succinyl coenzyme A. J Am Chem Soc 75: 2520.
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–85.
Söhling B, Gottschalk G (1993) Purification and characterisation of a coenzyme-A-dependent succinate-semialdehyde dehydrogenase from Clostridium kluyveri. Eur J Biochem 212: 121–127.
Thauer RK, Jungermann K, Henninger H, Wenning J, Decker K (1968) The energy metabolism of Clostridium kluyveri. Eur J Biochem 4: 173–180.
Weimer PJ (1984) Control of product formation during glucose fermentation by Bacillus macerans. J Gen Microbiol 130: 103–111.
Wolff RA, Urban G, O'Herrin SM, Keanealy WR (1992) A new anaerobic pathway for succinate degradation in Clostridium kluyveri (abstr O-32). In: Morello JA, Domer JE (eds) Abstr 92nd Annu Meet Am Soc Microbiol 1992. American Society for Microbiology, Washington DC, p 314.
Wolff RA, Urban G, O'Herrin SM, Keanealy WR (1993) Dehydrogenases involved in the conversion of succinate to 4-hydroxybutanoate by Clostridium kluyveri. Appl Environ Microbiol 59: 1876–1882.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Scherf, U., Söhling, B., Gottschalk, G. et al. Succinate-ethanol fermentation in Clostridium kluyveri: purification and characterisation of 4-hydroxybutyryl-CoA dehydratase/vinylacetyl-CoA Δ3-Δ2-isomerase. Arch. Microbiol. 161, 239–245 (1994). https://doi.org/10.1007/BF00248699
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00248699