Abstract
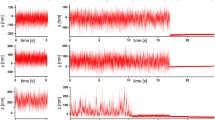
Lipid analogues and glycosylphosphati-dylinositol (GPI)-anchored proteins incorporated in glass-supported phospholipid bilayers (SBL) were coupled to small (30 nm diameter) fluorescent beads whose motion in the liquid phase was tracked by intensified fluorescence video microscopy. Streptavidin (St), covalently attached to the carboxyl modified surface of the polystyrene bead, bound either the biotinylated membrane component, or a biotinylated monoclonal antibody (mAb) directed against a specific membrane constituent. The positions of the beads tethered to randomly diffusing membrane molecules were recorded at 0.2 sec intervals for about l min. The mean square displacement (ϱ) of the beads was found to be a linear function of diffusion time t, and the diffusion coefficient, D, was derived from the relation, ϱ(t) = 4Dt. The values of D for biotinylated phosphatidylethanolamine (Bi-PE) dispersed in an egg lecithin: cholesterol (80:20%) bilayer obtained by this methodology range from 0.05 to 0.6 μm2/sec with an average of 〈D〉 = 0.26 μm2/sec, similar to the value of 〈D〉 = 0.24 μm2/sec for fluorescein-conjugated phosphati-dylethanolamine (Fl-PE) linked to St-coupled beads by the anti-fluorescein mAb 4-4-20 or its Fab fragment. These values of D are comparable to those reported for Fl-PE linked to 30 nm gold particles but are several times lower than that of Fl-PE in the same planar bilayer as measured by fluorescence photobleaching recovery, D = 1.3 μm2sec. The mobilities of two GPI-anchored proteins in similar SBL were also determined by use of the appropriate biotinylated mAb and were found to be 〈D〉 = 0.25 and 0.56 μm2/sec for the decay accelerating factor (DAF, CD55) and the human FcγRIIIB (CD16) receptors, respectively. The methodology described here is suitable for tracking any accessible membrane component.
Similar content being viewed by others
References
Ahlers, M., Grainger, D.W., Herron, J.N., Lim, K., Ringsdorf, H., Salesse, C. 1992. Quenching of fluorescein-conjugated lipids by antibodies. Biophys. J. 63:823–838
Anderson, C.M., Georgiou, G.N., Morrison, I.E.G., Stevenson, G.V.W., Cherry, R.J. 1992. Tracking of cell surface receptors by fluorescence digital imaging microscopy using a charge-coupled device camera. Low density lipoprotein and influenza virus receptor mobility at 4 C. J. Cell Sci. 101:415–425
Axelrod, D. 1983. Lateral motion of membrane proteins and biological function. J. Membrane Biol. 75:1–10
Axelrod, D., Koppel, D., Schlessinger, J., Elson, E., Webb, W.W. 1976. Mobility measurements by analysis of fluorescence recovery after photobleaching. Biophys. J. 16:1055–1069
Berg, H.C. 1983. Random Walks in Biology. Princeton University Press, Princeton, NJ
Bretscher, M.S. 1984. Endocytosis: relation to capping and cell locomotion. Science 224:681–686
Curtain, C.C., Gordon, L.M., Aloia, R.C. 1988. Lipid domains in biological membranes: Conceptual development and significance. In: Lipid Domains and the Relationship to Membrane Function. R.C. Aloia, C.C. Curtain, and L.M. Gordon, editors. pp. 1–15. Alan R. Liss, New York
Davitz, M.A., Schlesinger D., Nussenzweig, V. 1987. Isolation of decay accelerating factor (DAF) by a two-step procedure and determination of its N-terminal sequence. J. Imm. Methods 97:71–76
De Beus, A., Eisinger, J. 1992. Modulation of lateral transport of membrane components by spatial variations in diffusivity and solubility. Biophys. J. 63:607–615
DeBrabander, M., Nuydens, R., Ishihara, A., Holifield, B., Jacobson, K., Geerts, H. 1991. Lateral diffusion and retrograde movements of individual cell surface components on single motile cells observed with nanovid microscopy. J. Cell Biol. 112:111–124
Edidin, M., Kuo, S.C., Sheetz, M.P. 1991. Lateral movements of membrane glycoproteins restricted by dynamic cytoplasmic barriers. Science 254:1379–1382
Edidin, M., Stroynowski, I. 1991. Differences between the lateral organization of conventional and inositol phospholipidanchored membrane proteins. A further definition of micrometer scale membrane domains. J. Cell Biol. 112:1143–1150
Edwards, C., Frisch, H.L. 1976. A model for the localization of acetylcholine receptors at the muscle endplate. J. Neurobiol. 7:377–381
Einstein, A. 1905. Ueber die von der molecularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Ann. d. Physik 17:549–560
Eisinger, J., Flores, J., Petersen, W.P. 1986. A milling crowd model for local and long-range obstructed lateral diffusion—Mobility of excimeric probes in the membrane of intact erythrocytes. Biophys. J. 49:987–1001
Eisinger, J., Halperin, B.I. 1986. Effects of spatial variation in membrane diffusibility and solubility on the lateral transport of membrane components. Biophys. J. 50:513–521
Fahey, P.F., Webb, W.W. 1978. Lateral diffusion in phospholipid bilayer membranes and multilamellar liquid crystals. Biochemistry 17:3046–3053
Fleit, H.B., Wright, S.D., Unkeless, J.C. 1982. Human neutrophil Fc gamma receptor distribution and structure. Proc. Natl. Acad. Sci. USA 79:3275–3279
Geerts, H., De Brabander, M., Nuydens, R., Geuens, S., Moeremans, M., De Mey, J. Hollenbeck, P. 1987. Nanovid tracking: A new automatic method for the study of mobility in living cells based on colloidal gold and video microscopy. Biophys. J. 52:775–782
Ghosh, R.N., Webb, W.W. 1990. Evidence for intra-membrane constraints to cell surface LDL receptor motion. Biophys. J. 57:286a
Gross, D.J., Webb, W.W. 1988. Cell surface clustering and mobility of the liganded LDL receptor measured by digital video fluorescence microscopy. In: Spectroscopic Membrane Probes. L.M. Loew, editor pp. 19–45 CRC, Boca Raton, FL
Haverstick, D.M., Glaser, M. 1988. Visualization of domain formation in the inner and outer leaflets of a phospholipid bilayer. J. Cell Biol. 106:1885–1896
Keller, H.E. 1989. Objective lenses for confocal microscopy. In: The Handbook of Biological Confocal Microscopy. J. Pawley, editor. pp. 69–77. IMR, Madison, WI
Kinoshita, T., Medof, M.E., Silber, R., Nussenzweig, V. 1985. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J. Exp. Med. 162:75–92
Kranz, D.M., Herron, J.N, Voss, E.W., Jr. 1982. Mechanisms of ligand binding by monoclonal anti-fluorescyl antibodies. J. Biol. Chem. 257:6987–6995
Lee, G.M., Ishihara, A., Jacobson, K.A. 1991. Direct observation of Brownian motion of lipids in a membrane. Proc. Natl. Acad. Sci. USA 88:6274–6278
Low, M.G. 1989. The glycosyl-phosphatidylinositol anchor of membrane proteins. Biochim. Biophys. Acta 988:427–454
Petrossian, A., Owicki, J.C. 1984. Interaction of antibodies with liposomes bearing fluorescent haptens. Biochim. Biophys. Acta 776:217–227
Qian, H., Sheetz, M.P., Elson, E.L. 1991. Single particle tracking—Analysis of diffusion and flow in two-dimensional systems. Biophys. J. 60:910–921
Rodgers, W., Glaser, M. 1991. Characterization of lipid domains in erythrocyte membranes. Proc. Nat. Acad. Sci. USA 88:1364–1368
Rubenstein, J.L.R., Smith, B.A., McConnell, H.M. 1979. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc. Natl. Acad. Sci. USA 76:15–18
Sassaroli, M., Vauhkonen, M., Perry, D., Eisinger, J. 1990. The lateral diffusivity of lipid analogue excimeric probes in dimyristoylphosphatidylcholine bilayers. Biophys. J. 57:281–290
Scalettar, B.A., Abney, J.R. 1991. Molecular crowding and protein diffusion in biological membranes. Comments Mol. Cell. Biophys. 7:79–107
Singer, S.J., Nicolson, G.L. 1972. The fluid mosaic model of the structure of cell membranes. Science 175:720–731
Taylor, R.B., Duffus, P.H., Raff, M.C., de Petris, S. 1971. Redistribution and pinocytosis of lymphocyte surface immunoglobulin molecules induced by anti-immunoglobulin antibody. Nature New Biol. 233:225–229
Thomas, J., Webb, W., Davitz, M.A., Nussenzweig, V. 1987 Decay accelerating factor diffuses rapidly on HeLa(AE) cell surfaces. Biophys. J. 51:522a
Yechiel, E., Edidin, M. 1987. Micrometer-scale domains in fibroblast plasma membranes. J. Cell Biol. 105:755–760
Zhang, F., Schmidt, W.G., Hou, Y., Williams, A.F., Jacobson, K. 1992. Spontaneous incorporation of the glycosyl-phosphatidylinositol-linked protein Thy-1 into cell membranes. Proc. Natl. Acad. Sci. USA 89:5231–5235
Author information
Authors and Affiliations
Additional information
This work was supported by National Institutes of Health grants 1R24 RR05272 and AI-24322.
Rights and permissions
About this article
Cite this article
Fein, M., Unkeless, J., Chuang, F.Y.S. et al. Lateral mobility of lipid analogues and GPI-anchored proteins in supported bilayers determined by fluorescent bead tracking. J. Membarin Biol. 135, 83–92 (1993). https://doi.org/10.1007/BF00234654
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00234654