Summary
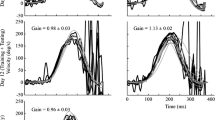
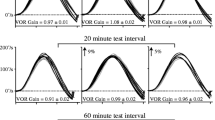
We habituated the dominant time constant of the horizontal vestibuloocular reflex (VOR) of rhesus and cynomolgus monkeys by repeated testing with steps of velocity about a vertical axis and adapted the gain of the VOR by altering visual input with magnifying and reducing lenses. After baseline values were established, the nodulus and ventral uvula of the vestibulocerebellum were ablated in two monkeys, and the effects of nodulouvulectomy and flocculectomy on VOR gain adaptation and habituation were compared. The VOR time constant decreased with repeated testing, rapidly at first and more slowly thereafter. The gain of the VOR was unaffected. Massed trials were more effective than distributed trials in producing habituation. Regardless of the schedule of testing, the VOR time constant never fell below the time constant of the semicircular canals (≈5 s). This finding indicates that only the slow component of the vestibular response, the component produced by velocity storage, was habituated. In agreement with this, the time constant of optokinetic after-nystagmus (OKAN) was habituated concurrently with the VOR. Average values for VOR habituation were obtained on a per session basis for six animals. The VOR gain was adapted by natural head movements in partially habituated monkeys while they wore ×2.2 magnifying or ×0.5 reducing lenses. Adaptation occurred rapidly and reached about ±30%, similar to values obtained using forced rotation. VOR gain adaptation did not cause additional habituation of the time constant. When the VOR gain was reduced in animals with a long VOR time constant, there were overshoots in eye velocity that peaked at about 6–8 s after the onset or end of constant-velocity rotation. These overshoots occurred at times when the velocity storage integrator would have been maximally activated by semicircular canal input. Since the activity generated in the canals is not altered by visual adaptation, this finding indicates that the gain element that controls rapid changes in eye velocity in the VOR is separate from that which couples afferent input to velocity storage. Nodulouvulectomy caused a prompt and permanent loss of habituation, returning VOR time constants to initial values. VOR gain adaptation, which is lost after flocculectomy, was unaffected by nodulouvulectomy. Flocculectomy did not alter habituation of the VOR or of OKAN. Using a simplified model of the VOR, the decrease in the duration of vestibular nystagmus due to habituation was related to a decrement in the dominant time constant of the velocity storage integrator (1/h 0). Nodulouvulectomy, which reversed habituation, would be effected by decreasing h 0, thereby increasing the VOR time constant. Small values of h 0 would cause velocity storage to approach an ideal integrative process, leading the system to become unstable. By controlling the VOR time constant through habituation, the nodulus and uvula can stabilize the slow component of the VOR. VOR gain adaptation was related to a modification of the direct vestibular path gain g 1, without altering the coupling to velocity storage g 0 or its time constant (1/h 0). The mismatched direct- and indirect-pathway gains simulated the overshoots in the dynamic response to a step in velocity, that were observed experimentally. We conclude that independent distributed elements in the VOR modify its dynamic response, under control of separate parts of the vestibulocerebellum.
Similar content being viewed by others
References
Benson AJ (1974) Modification of the response to angular accelerations by linear accelerations. In: Kornhuber HH (eds) Handbook of Sensory Physiology, vol VI/2. Springer, Berlin, pp 281–320
Brandt T, Daroff RB (1980) Physical therapy for benign paroxysmal positional vertigo. Arch Otolaryngol 106: 484–485
Bronstein AM, Gresty MA (1991) Compensatory eye movements in the presence of conflicting canal and otolith signals. Exp Brain Res 85: 697–700
Buizza A, Courjon JH, Flandrin JM, Schmid R (1988) Cross-coupled effects of repeated vestibular and optokinetic stimulations on the dynamics of the vestibulo-ocular and optokinetic reflexes in the cat. Exp Brain Res 70: 215–251
Büttner U, Waespe W, Henn V (1976) Duration and direction of optokinetic after-nystagmus as a function of stimulus exposure time in the monkey. Arch Psychiat Nervenkr 222: 281–291
Clement G, Courjon JH, Jeannerod M, Schmid R (1981) Unidirectional habituation of vestibulo-ocular responses by repeated rotational or optokinetic stimulations in the cat. Exp Brain Res 42: 34–42
Cohen B, Helwig D, Raphan T (1987) Baclofen and velocity storage: a model of the effects of drug on the vestibulo-ocular reflex in the rhesus monkey. J Physiol (Lond) 393: 703–725
Cohen B, Matsuo V, Raphan T (1977) Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. J Physiol (Lond) 270: 321–344
Collewijn H, Martins AJ, Steinman RM (1983) Compensatory eye movements during active and passive head movements: fast adaptation to changes in visual magnification. J Physiol (Lond) 340: 259–286
Collewijn H, Winterson BJ, Van der Steen J (1980) Post-rotatory nystagmus and optokinetic after-nystagmus in the rabbit: linear rather than exponential decay. Exp Brain Res 40: 330–338
Collins WE (1974) Habituation of vestibular responses with and without visual stimulation. In: Kornhuber HH (eds) Handbook of Sensory Physiology vol VI/2. Springer, Berlin, pp 369–388
Crampton GH (1962) Directional imbalance of vestibular nystagmus in cat following repeated unidirectional angular acceleration. Acta Otolaryngol (Stockh) 55: 41–48
Crampton GH (1964) Habituation of ocular nystagmus of vestibular origin. In: Bender MB (eds) The oculomotor system. Harper and Row, New York, pp 332–346
Dai MJ, Raphan T, Cohen B (1991) Spatial orientation of the vestibular system: dependence of optokinetic after-nystagmus (OKAN) on gravity. J Neurophysiol 66: 1422–1439
Demer JL (1981) The variable gain element of the vestibulo-ocular reflex is common to the optokinetic system of the cat. Brain Res 229: 1–13
Dodge R (1923) Habituation to rotation. J Exp Psychol 6: 1–35
Goldberg JM, Fernandez C (1971) Physiology of the peripheral neurons innervating semicircular canal of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J Neurophysiol 34: 635–660
Gonshor A, Melvill Jones G (1976a) Short-term adaptive changes in the human vestibulo-ocular reflex arc. J Physiol (Lond) 256: 361–379
Gonshor A, Melvill Jones G (1976b) Extreme vestibulo-ocular adaptation induced by prolonged optical reversal of vision. J Physiol (Lond) 256: 381–414
Guedry FE (1964) Visual control of habituation to complex vestibular stimulation in man. Acta Otolaryngol (Stockh) 58: 377–389
Hain TC, Zee DS, Maria BL (1988) Tilt suppression of VOR in patients with cerebellar lesions. Acta Otolaryngol (Stockh) 105: 13–20
Halmagyi GM, Rudge P, Gresty MA, Leign RJ, Zee DS (1980) Treatment of periodic alternating nystagmus. Ann Neurol 8: 609–611
Halstead W, Yacorzynski G, Fearing F (1937) Further evidence of cerebellar influence in the habituation of after-nystagmus in pigeons. Am J Physiol 120: 350–355
Harrison REW, Baker JF, Isu N, Wickland CR, Peterson BW (1986) Dynamics of adaptive change in vestibulo-ocular reflex directions. I. Rotations in the horizontal plane. Brain Res 371: 162–165
Hood JD, Pfaltz CR (1954) Observations upon the effects of repeated stimulation upon rotational and caloric nystagmus. J Physiol (Lond) 124: 130–144
Ito M (1982) Cerebellar control of the vestibulo-ocular reflex — around the flocculus hypothesis. Ann Rev Neurosci 5: 275–296
Ito M (1984) The cerebellum and neural control. Raven Press, New York
Jäger J, Henn V (1981) Vestibular habituation in man and monkey during sinusoidal rotation. Ann NY Acad Sci 374: 330–339
Jeannerod M, Clement G, Courjon JH (1981) Unilateral habituation of vestibulo-ocular responses in the cat. Ann NY Acad Sci374: 340–351
Jeannerod M, Magnin M, Schmid R, Stefanelli M (1976) Vestibular habituation to angular velocity steps in the cat. Biolog Cyber 22: 39–48
Katz E, DeJong JMBV, Büttner-Ennever JA, Cohen B (1991) Effects of midline medullary lesions on velocity storage and the vestibulo-ocular reflex. Exp Brain Res 87: 505–520
Leigh RJ, Robinson DA, Zee DS (1981) A hyptothetical explanation for periodic alternating nystagmus: instability in the optokinetic-vestibular system. Ann NY Acad Sci 374: 619–635
Lisberger SG (1988) The neural basis for learning simple motor skills. Science 242: 728–735
Lisberger SG, Miles FA, Optican LM (1983) Frequency-selective adaptation: evidence for channels in the vestibulo-ocular reflex? J Neurosci 6: 1234–1244
Lisberger SG, Miles FA, Optican LM, Eighmy BB (1981) Optokinetic response in monkey: underlying mechanisms and their sensitivity to long-term adaptive changes in vestibuloocular reflex. J Neurophysiol 45: 869–890
Lisberger SG, Miles FA, Zee DS (1984) Signals used to compute errors in monkey vestibuloocular reflex: possible role of flocculus. J Neurophysiol 52: 1140–1153
Lisberger SG, Pavelko TA (1988) Brain stem neurons in modified pathways for motor learning in the primate vestibulo-ocular reflex. Science 242: 771–773
Mason CR, Baker JF (1989) Effects of optokinetic velocity and medial vestibule-cerebellum lesions on vestibulo-ocular reflex direction adaptation in the cat. Brain Res 490: 373–377
Melvill Jones G (1985) Adaptive modulation of VOR parameters by vision. In: Berthoz A, Melvill Jones G (eds) Adaptive Mechanisms in Gaze Control. Elsevier, Amsterdam, pp 21–50
Melvill Jones G, Berthoz A, Segal B (1984) Adaptive modification of the vestibulo-ocular reflex by mental effort in darkness. Exp Brain Res 56: 149–153
Miles FA, Braitman DJ (1980) Long-term adaptive changes in vestibuloocular reflex. II. Electrophysiological observations on semicircular canal primary afferents. J Neurophysiol43: 1426–1436
Miles FA, Eighmy BB (1980) Long-term adaptive changes in primate vestibuloocular reflex. I. Behavioral observations. J Neurophysiol 43: 1406–1425
Miles FA, Lisberger SG (1981) Plasticity in the vestibulo-ocular reflex: a new hypothesis. Ann Rev Neurosci 4: 273–299
Raphan T, Cohen B, Henn V (1981) Effects of gravity on rotatory nystagmus in monkeys. Ann NY Acad Sci 374: 44–55
Raphan T, Matsuo V, Cohen B (1979) Velocity storage in the vestibulo-ocular reflex arc. Exp Brain Res 35: 229–248
Raphan T, Sturm D (1991) Modeling the spatiotemporal organization of velocity storage in the vestibuloocular reflex by optokinetic studies. J Neurophysiol 66: 1410–1421
Schmid R, Jeannerod M (1985) Vestibular habituation: an adaptive process? In: Berthoz A, Melvill Jones G (eds) Adaptive Mechanisms in Gaze Control. Elsevier, Amsterdam, pp 113–132
Schuknecht HF (1969) Cupulolithiasis. Arch Otolaryngol 90: 765–778
Schultheis LW, Robinson DA (1981) Directional plasticity of the vestibulo-ocular reflex in the cat. Ann NY Acad Sci 374: 504–512
Singleton GT (1967) Relationships of the cerebellar nodulus to vestibular function: a study of the effects of nodulectomy on habituation. Laryng 77: 1579–1620
Skavenski AA, Blair SM, Westheimer G (1981) The effect of habituating vestibular and optokinetic nystagmus on each other. J Neurosci 1: 351–357
Solomon D, Cohen B (1992a) Stabilization of gaze during circular locomotion in light. I. Compensatory head and eye nystagmus in the running monkey. J Neurophysiol 67: 1146–1157
Solomon D, Cohen B (1992b) Stabilization of gaze during circular locomotion in darkness. II. Contribution of velocity storage to compensatory eye and head nystagmus in the running monkey. J Neurophysiol 67: 1158–1170
Solomon D, Raphan T, Cohen B (1985) Effects of cerebellar stimulation on velocity storage in the monkey. Soc Neurosci Abstr 11: 693
Stratton GM (1897) Vision without inversion of the retinal image. Psychol Rev 4: 341–360, and 463–481
Thorpe WH (1956) Learning and Instinct in Animals. Harvard University Press, Cambridge
Viirre E, Tweed D, Milner K, Vilis T (1986) A reexamination of the gain of the vestibuloocular reflex. J Neurophysiol 56: 439–450
Waespe W, Cohen B, Raphan T (1983) Role of the flocculus and paraflocculus in optokinetic nystagmus and visual-vestibular interactions: effects of lesions. Exp Brain Res 50: 9–33
Waespe W, Cohen B, Raphan T (1985) Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science 228: 199–201
Young LR, Henn V (1976) Selective habituation of vestibular nystagmus by visual stimulation in the monkey. Acta Otolaryngol (Stockh) 82: 165–171
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cohen, H., Cohen, B., Raphan, T. et al. Habituation and adaptation of the vestibuloocular reflex: a model of differential control by the vestibulocerebellum. Exp Brain Res 90, 526–538 (1992). https://doi.org/10.1007/BF00230935
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00230935