Summary
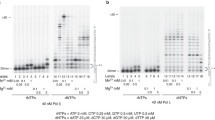
The effect of aflatoxin Bt (AFB,) on the template function for RNA synthesis of several single and double-stranded synthetic DNAs containing cytosine (C) and/or hypoxanthine (H) bases is studied in vitro. The results indicate that AFB,, after liver microsome activation, strongly inhibits the template function of poly[d(I-C)] and has little, if any, effect on polydI . polydC, polydI, or polydC. This conclusion is reached whether rat liver nuclear free RNA polymerase or E. coli RNA polymerase is used for the transcription. The mechanism of this inhibition is believed mainly due to the inhibition of elongation of RNA synthesis, because autoradiography of the [α-32 P]GTP labeled RNAs after polyacrylamide gel electrophoresis clearly shows that the size of the RNA from AFB1 treated group is dramatically reduced. The evidence that the selective inhibition of poly[d(I-C)] template function is a direct reflection of the binding of AFB1 to poly[d(I-C)] is provided by the use of radioactive [3H]AFB1 for the binding and by spectrum analysis of the appearance of a broad AFB1-DNA adduct peak between 300 nm and 400 nm right after the typical DNA peak at 260 nmn. These data, which are in direct support to our recent report (F.L. Yu, et al., Carcinogenesis, 11, 475–478, 1990), suggest that the binding of AFB1 prefers alternating, double-stranded DNA, and the binding affinity of AFB1 to DNA is greatly reduced when the bases are in either single- or double-stranded homopolymer forms. Furthermore, since AFB1 binds to and inhibits the template function of poly[d(I-C)], these results also suggest that the binding of AFB1 to DNA may not be exclusively limited to guanine as previously assumed.
Similar content being viewed by others
References
McCann J, Choi E, Yamasaki E, Ames BN: Detection of Carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proc Natl Acad Sci USA 72: 5135–5139, 1975
Ames BN, Magaw R, Gold LS: Ranking possible carcinogenic hazards. Science 236: 271–280, 1987
Wogan GN: Aflatoxin carcinogenesis. In: H Busch (ed.) Methods in Cancer Res 7. Academic Press, New York, 1973, pp 309–345
Busby WF, Wogan GN: Aflatoxins. In: CE Searle (ed.) Chemical Carcinogens, 2nd ed., ACS Monograph 182, American Chemical Society, Washington DC, 1984, pp 945–1136
Lijinsky W: A view of the relation between carcinogenesis and mutagenesis. Environmental Molecular Mutagenesis 14 (Suppl 16): 78–84, 1989
Miller EC, Miller JA: Searches for the ultimate chemical carcinogens and their reactions with cellular macromolecules. Cancer 47: 2327–2345, 1981
Lin JK, Miller JA, Miller EC: 2–3-Dihydro-2-(guan-7-yl)-3-hydroxy-aflatoxin B1 — a major acid hydrolysis product of aflatoxin B1-DNA or ribosomal RNA adducts formed in hepatic microsome-mediated reactions and in rat liver in vivo. Cancer Res 37: 4430–4438, 1977
Essigmann JM, Croy RG, Nadzan AM, Busby WF Jr, Reinhold VN, Busci G, Wogan GN: Structure identification of the major DNA adduct formed by aflatoxin B1 in vitro. Proc Natl Acad Sci USA 74: 1870–1874, 1977
Martin CN, Garner RC: Aflatoxin-oxide generated by chemical or enzymic oxidation of aflatoxin B1 causes guanine substitution in nucleic acids. Nature 267: 863–865, 1977
Yu FL: Mechanism of aflatoxin B1 inhibition of rat hepatic nuclear RNA synthesis. J Biol Chem 252: 3245–3251, 1977
Yu FL: Studies on the mechanisms of aflatoxin B1 inhibition of rat liver nucleolar RNA synthesis. J Biol Chem 256: 3292–3297, 1981
Yu FL: Preferential binding of aflatoxin B1 to the transcriptionally active regions of rat liver nucleolar chromatin in vivo and in vitro. Carcinogenesis 4: 889–893, 1983
Yu FL, Geronimo IH, Bender W, Permthamsin J: Correlation studies between the binding of aflatoxin B1 to chromatin components and the inhibition of RNA synthesis. Carcinogenesis 9: 527–532, 1988
Yu FL, Bender W, Geronimo III: The binding of aflatoxin B1 to rat liver nuclear proteins and its effects on DNAdependent RNA synthesis. Carcinogenesis 9: 533–540, 1988
Yu FL, Bender W, Geronimo IH: Base and sequence specificities of aflatoxin B, binding to single- and doublestranded DNAs. Carcinogenesis 11: 475–478, 1990
Yu FL: Two functional states of the RNA polymerases in the rat hepatic nuclear and nucleolar fractions. Nature 251: 344–346, 1974
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yu, FL., Bender, W. & Wu, Z. Transcriptional effect of aflatoxin B1 on cytosine and/or hypoxanthine containing DNAs. Mol Cell Biochem 103, 1–8 (1991). https://doi.org/10.1007/BF00229587
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00229587