Abstract
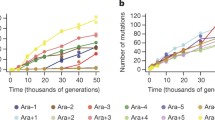
The basic structure and properties of Ty elements are considered with special reference to their role as agents of evolutionary change. Ty elements may generate genetic variation for fitness by their action as mutagens, as well as by providing regions of portable homology for recombination. The mutational spectra generated by Ty 1 transposition events may, due to their target specificity and gene regulatory capabilities, possess a higher frequency of adaptively favorable mutations than spectra resulting from other types of mutational processes. Laboratory strains contain between 25–35 elements, and in both these and industrial strains the insertions appear quite stable. In contrast, a wide variation in Ty number is seen in wild isolates, with a lower average number/genome. Factors which may determine Ty copy number in populations include transposition rates (dependent on Ty copy number and mating type), and stabilization of Ty elements in the genome as well as selection for and against Ty insertions in the genome. Although the average effect of Ty transpositions are deleterious, populations initiated with a single clone containing a single Ty element steadily accumulated Ty elements over 1,000 generations. Direct evidence that Ty transposition events can be selectively favored is provided by experiments in which populations containing large amounts of variability for Ty1 copy number were maintained for ∼100 generations in a homogeneous environment. At their termination, the frequency of clones containing 0 Ty elements had decreased to ∼0.0, and the populations had became dominated by a small number of clones containing >0 Ty elements. No such reduction in variability was observed in populations maintained in a structured environment, though changes in Ty number were observed. The implications of genetic (mating type and ploidy) changes and environmental fluctuations for the long-term persistence of Ty elements within the S. cerevisiae species group are discussed.
Similar content being viewed by others
References
Adams, J. & P. W. Oeller, 1986. Structure of evolving populations of Saccharomyces cerevisiae: Adaptive changes are frequently associated with alterations involving mobile elements belonging to the Ty family. Proc. Natl. Acad. Sci. USA 83: 7124–7127.
Adams, S. E., J. Mellor, K. Gull, R. B. Sim, M. F. Tuite, S. M. Kingsman & A. J. Kingsman, 1987. The functions and relationships of Ty-VLP proteins in yeast reflect those of mammalian retroviral proteins. Cell 49: 111–119.
Barnett, J. A., R. W. Payne & D. Yarrow, 1983. Yeasts, characteristics and identification. Cambridge University Press, Cambridge.
Berg, D. E. & M. M. Howe, 1989. eds. Mobile DNA. American Society for Microbiology, Washington, D.C.
Boeke, J. D., 1989. Transposable elements in Saccharomyces cerevisiae, pp. 335–374 in Mobile DNA, edited by D. E. Berg and M. M. Howe. American Society for Microbiology, Washington D.C.
Boeke, J. D., D. J. Eichinger, D. Castrillon & G. R. Fink, 1988. The Saccharomyces cerevisiae genome contains functional and non-functional copies of transposon Ty1. Mol. Cell. Biol. 8: 1432–1442.
Boeke, J. D., D. J. Eichinger & G. Natsoulis, 1991. Doubling Ty1 element copy number in Saccharomyces cerevisiae: host genome stability and phenotypic effects. Genetics 129: 1043–1052.
Boeke, J. D. & D. J. Garfinkel, 1988. Yeast Ty elements as retroviruses, pp. 15–39 in Viruses of Fungi and Simple Eukaryotes, edited by Y. Koltin and M. J. Leibowitz. Marcel Dekker Inc, New York.
Boeke, J. D., D. J. Garfinkel, C. A. Styles & G. R. Fink, 1985. Ty elements transpose through an RNA intermediate. Cell 40: 491–500.
Boeke, J. D., C. A. Styles & G. R. Fink, 1986. Saccharomyces cerevisiae SPT3 gene is required for transposition and transpositional recombination of chromosomal Ty elements. Mol. Cell. Biol. 6: 3575–3581.
Cameron, J. R., E. Loh & R. W. Davis, 1979. Evidence for transposition of dispersed repetitive DNA families in yeast. Cell 16: 739–751.
Chao, L., C. Vargas, B. B. Spear & E. C. Cox, 1983. Transposable elements as mutator genes in evolution. Nature 303: 633–635.
Chao, L. & S. M. McBroom, 1985. Evolution of transposable elements: An IS10 insertion increases fitness in E. coli. Mol. Biol. Evol. 2: 359–369.
Charlesworth, B. & C. H. Langley, 1989. The population genetics of Drosophila transposable elements. Annu. Rev. Genet. 23: 251–287.
Clare, J. J., M. Belcourt & P. J. Farabaugh, 1988. Efficient translational frameshifting occurs within a conserved sequence of the overlap between the two genes of a yeast Ty 1 transposon. Proc. Natl. Acad. Sci. USA 85: 6816–6820.
Clare, J. J. & P. J. Farabaugh, 1985. Nucleotide sequence of a yeast Ty element: evidence for an unusual mechanism of gene expression. Proc. Natl. Acad. Sci. USA 82: 2829–2833.
Clark, D. J., V. W. Bilanchone, L. J. Haywood, S. L. Dildine & S. B. Sandmeyer, 1988. A yeast sigma composite element, Ty3, has properties of a retrotransposon. J. Biol. Chem. 263: 1413–1423.
Conover, W. J., 1971. Practical Nonparametric Statistics. John Wiley, New York.
Cornelis, G., 1980. Transposition of Tn951 (Tnlac) and cointegrate formation are thermosensitive processes. J. Gen. Microbiol. 117: 243–247.
Curcio, M. J., N. J. Sanders & D. J. Garfinkel, 1988. Transpositional competence and transcription of endogenous Ty elements in Saccharomyces cerevisiae: implications for regulation of transposition. Mol. Cell. Biol. 8: 3571–3581.
Doolittle, W. F. & C. Sapienza, 1980. Selfish genes, the phenotype paradigm and genome evolution. Nature 284: 601–603.
Eibel, H., J. Gafner, A. Stotz & Philippsen, 1980. Characterization of the yeast mobile element Ty1. Cold Spring Harbor Symp. Quant. Biol. 45: 609–617.
Eibel, H. & P. Philippsen, 1984. Preferential integration of yeast transposable element Ty into a promoter region. Nature 307: 386–388.
Elder, R. T., T. P.St John, D. T. Stinchcomb & R. W. Davis, 1980. Studies on the transposable element Tyl of yeast. I. RNA homologous to Ty1. Cold Spring Harbor Symp. Quant. Biol. 45: 581–584.
Elder, R. T., E. Loh & R. W. Davis, 1983. RNA from the yeast transposable element Ty1 has both ends in the direct repeats, a structure similar to retrovirus RNA. Proc. Natl. Acad. Sci. USA 80: 2432–2436.
Errede, B., T. S. Cardillo, F. Sherman, E. Dubois, J. Deschamps & J. Wiame, 1980. Mating signals control expression of mutations resulting from insertion of a transposable repetitive element adjacent to diverse yeast genes. Cell 22: 427–436.
Farabaugh, P. J. & G. R. Fink, 1980. Insertion of the eukaryotic transposable element Ty1 creates a 5 bp duplication. Nature 286: 352–356.
Fink, G. R., J. D. Boeke & D. J. Garfinkel, 1986. The mechanism and consequences of retrotransposition. Trends Genet. 2: 118–123.
Finnegan, D. J., 1989. Eukaryotic transposable elements and genome evolution. Trends Genet. 5: 103–107.
Fitzpatrick, B. J. & J. Sved, 1986. High levels of fitness modifiers induced by hybrid dysgenesis in Drosophila melanogaster. Genet. Res. 48: 89–94.
Gafner, J., E. M.De Robertis & P. Philippsen, 1983. Delta sequences in the 5′ region of yeast tRNA genes. EMBO J. 2: 583–591.
Gatner, J. & P. Philippsen, 1980. The yeast transposon Ty1 generates duplicates of target DNA in insertion. Nature 286: 414–418.
Garfinkel, D. J., J. D. Boeke & G. R. Fink, 1985. Ty element transposition: reverse transcriptase and virus-like particles. Cell 42: 507–517.
Garfinkel, D. J. & J. N. Strathern, 1991. Ty mutagenesis in Saccharomyces cerevisiae Meth. Enzymol. 194: 342–361.
Giroux, C. N., J. R. A. Mis, M. K. Pierce, S. E. Kohalmi & B. A. Kunz, 1988. DNA sequence analysis of spontaneous mutations in the SUP4-o gene of Saccharomyces cerevisiae. Mol. Cell. Biol. 8: 978–981.
Goebl, M. G. & T. D. Petes, 1986. Most of the yeast genomic sequences are not essential for cell growth and division. Cell 46: 983–992.
Hall, B. G., 1988. Adaptive evolution that requires multiple spontaneous mutations. I. Mutations involving an insertion sequence. Genetics 120: 887–897.
Hall, B. G., 1990. Spontaneous point mutations that occur more often when advantageous than when neutral. Genetics 126: 5–16.
Hansen, L. J., D. L. Chalker & S. B. Sandmeyer, 1988. Ty3, a yeast retrotransposon associated with tRNA genes, has homology to animal retroviruses. Mol. Cell. Biol. 8: 5245–5256.
Hansen, L. J. & S. B. Sandmeyer, 1990. Characterization of a transpositionally active Ty3 element and identification of the Ty3 integrase protein. J. Virol. 64: 2599–2607.
Iida, H., 1988. Multistress resistance of Saccharomyces cerevisiae is generated by insertion of Ty into the 5′ coding region of the adenylate cyclase gene. Mol. Cell. Biol. 8: 5555–5560.
Kaplan, N. L., R. R. Hudson & C. H. Langley, 1989. The ‘hitch-hiking’ effect revisited. Genetics 123: 887–899.
Kingsman, A. J. & S. M. Kingsman, 1988. Ty: A retroelement moving forward. Cell 53: 333–335.
Kleckner, N., 1990. Regulation of transposition in bacteria. Annu. Rev. Cell Biol. 6: 297–327.
Klein, H. L. & T. D. Petes, 1984. Genetic mapping of Ty elements in Saccharomyces cerevisiae. Mol. Cell. Biol. 4: 329–339.
Koch, A. L., 1974. The pertinence of the periodic selection phenomenon to prokaryotic evolution. Genetics 77: 127–142.
Kondrashov, A. S. & J. F. Crow, 1991. Haploidy or diploidy: which is better? Nature 351: 314–315.
Kretschmer, P. J. & S. N. Cohen, 1977. Selected translocation of plasmid genes: frequency and regional specificity of translocation of the Tn3 element. J. Bacteriol. 130: 888–899.
Kurlandzka, A., R. F. Rosenzweig & J. Adams, 1991. Identification of adaptive changes in an evolving population of Escherichia coli: the role of regulatory changes with highly pleiotropic effects. Mol. Biol. Evol. 8: 261–281.
Laski, F. A., D. C. Rio & G. M. Rubin, 1986. Tissue specificity of Drosophila P element transposition is regulated at the level of mRNA splicing. Cell 44: 7–19.
Lewontin, R. C., 1974. The Genetic Basis of Evolutionary Change. Columbia University Press, NY.
Liebman, S. W. & S. Picologlou, 1988. Recombination associated with yeast retrotransposons, pp. 63–90 in Viruses of Fungi and Simple Eukaryotes, edited by Y. Koltin and M. J. Leibowitz. Marcel Dekker Inc., NY.
Lopilato, J. & A. Wright, 1990. Mechanisms of activation of the cryptic bgl operon of Escherichia coli K12, pp. 435–444 in The Bacterial Chromosome edited by K. Drlica and M. Riley. American Society of Microbiology Publications. Washington, D.C.
MacArthur, R. H., 1962. Some generalized theorems of natural selection. Proc. Natl. Acad. Sci. USA 231: 123–128.
Mackay, T. F. C., 1985. Transposable element-induced response to artificial selection in Drosophila melanogaster. Genetics 111: 351–374.
Mackay, T. F. C., 1986. Transposable element-induced fitness mutations in Drosophila melanogaster. Genet. Res. 48: 77–87.
Maimer, E., 1990. Taxonomie und Oekologie von Hefen aus Frucht und Fruchtzubereitungen. Unpublished Ph.D. thesis, Technische Universität München-Weihenstephan.
Maynard Smith, J. & J. Haigh, 1974. The hitch-hiking effect of a favourable gene. Genet. Res. 23: 25–35.
McClanahan, T. & K. McEntee, 1984. Specific transcripts are elevated in Saccharomyces cerevisiae in response to DNA damage. Mol. Cell. Biol. 4: 2356–2363.
McDonald, J. F., 1989. The potential evolutionary significance of retroviral-like transposable elements in peripheral populations, pp. 190–205, in Evolutionary Biology of Transient Unstable Populations, edited by A. Fontdevila, Springer-Verlag, New York.
McDonald, J. F., 1990. Macroevolution and retroviral elements. BioScience 40: 183–191.
Mellor, J., S. M. Fulton, W. W. Dobson, S. M. Kingsman & A. J. Kingsman, 1985a. A retrovirus-like strategy for expression of a fusion protein encoded by yeast transposon Ty1. Nature 313: 243–246.
Mellor, J., A. J. Kingsman & S. M. Kingsman, 1986. Ty, an endogenous retrovirus of yeast? Yeast 2: 145–152.
Mellor, J., M. H. Malim, K. Gull, M. F. Tiute, S. McCready, T. Dibbayawan, S. M. Kingsman & A. J. Kingsman, 1985b. Reverse transcriptase activity and Ty RNA are associated with virus-like particles in yeast. Nature 318: 583–586.
Misra, S. & D. C. Rio, 1990. Cytotype control of Drosophila P element transposition: The 66 kd protein is a repressor of transposase activity. Cell 62: 269–284.
Modi, R., L. H. Castilla, S. Puskas-Rozsa, R. B. Helling & J. Adams, 1992. Genetic changes accompanying increased fitness in evolving populations of Escherichia coli. Genetics 130: 241–249.
Morawetz, C., 1987. Effect of irradiation and mutagenic chemicals on the generation of ADH2-constitutive mutants in yeast. Significance for the inducibility of Ty transposition. Mut. Res. 177: 53–60.
Muller, F., K. H. Bruhl, K. Freidel, K. V. Kowallik & M. Ciriacy, 1987. Processing of Ty1 proteins and formation of Ty1 virus-like particles in Saccharomyces cerevisiae. Mol. Gen. Genet. 207: 421–429.
Natsoulis, G., W. Thomas, M. Roghmann, F. Winston & J. D. Boeke, 1989. Transposition in Saccharomyces cerevisiae is nonrandom. Genetics 123: 269–279.
Nevers, P., H. J. Reiff & H. Saedler, 1977. Mutations affecting IS1-mediated deletion formation in E. coli. In, DNA Insertion elements plasmids and episomes, pp. 125–128 edited by A. Bukhari, J. Shapiro and S. Adhya. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Orgel, L. E. & F. H. C. Crick, 1980. Selfish DNA: the ultimate parasite. Nature 284: 604–607.
Oyen, T. B. & O. S. Gabrielsen, 1983. Non-random distribution of the Ty1 elements within nuclear DNA of Saccharomyces cerevisiae. Fed. Eur. Bioch. Soc. 161: 201–206.
Paquin, C. E. & J. Adams, 1983. Frequency of fixation of adaptive mutations is higher in evolving diploid than haploid populations. Nature 302: 495–500.
Paquin, C. & V. M. Williamson, 1984. Temperature effects on the rate of Ty transposition. Science 226: 53–55.
Paquin, C. & V. M. Williamson, 1986. Ty insertions at two loci account for most of the spontaneous antimycin A resistance mutations during growth at 15°C of Saccharomyces cerevisiae strains lacking ADH1. Mol. Cell. Biol. 6: 70–79.
Pasyukova, E. G., E. S. Belyaeva, G. L. Kogan, L. Z. Kaidanov & V. A. Gvozdev, 1986. Concerted transpositions of mobile genetic elements coupled with fitness changes in Drosophila melanogaster. Mol. Biol. Evol. 3: 299–312.
Pasyukova, E. G., E. S. Belyaeva, L. E. Ilyinskaya & V. A. Gvozdev, 1988. Outcross-dependent transpositions of copia-like mobile genetic elements in chromosomes of an inbred Drosophila melanogaster stock. Mol. Gen. Genet. 212: 281–286.
Pedersen, M. B., 1985. DNA sequence polymorphisms in the genus Saccharomyces. II. Analysis of the genes RDN1, HIS4, LEU2 and Ty transposable elements in Carlsberg, Tuborg and 22 Bavarian brewing strains. Carlsberg Res. Commun. 50: 263–272.
Pedersen, M. B., 1986. DNA sequence polymorphisms in the genus Saccharomyces. III. Restricition endonuclease fragment patterns of chromosomal regions in brewing and other yeast strains. Carlsberg Res. Commun. 51: 163–183.
Pedersen, M. B., 1988. The use of nucleotide sequence polymorphisms and DNA karyotyping in the identification of brewer's yeast strains and in microbiological control, pp. 180–194 in Modern Methods of Plant Analysis, New Series Vol 7, Beer Analysis, edited by H. F. Linskens and J. F. Jackson. Springer-Verlag, NY.
Perrot, P., S. Richerd & M. Valéro, 1991. Transition from haploidy to diploidy. Nature 351: 315–317.
Philippsen, P., H. Eibel, J. Gafner & A. Stotz, 1983. Ty elements and the stability of the yeast genome, pp. 189–200 in Gene expression in Yeast. Proceedings of the Alko Yeast Symposium Helsinki, edited by M. Korhola and E. Vaisanen. Foundation for Biotechnical and Industrial Fermentation Research, Helsinki.
Picologlou, S., N. Brown & S. W. Liebman, 1990. Mutations in RAD6, a yeast gene encoding a ubiquitin-conjugating enzyme, stimulate retrotransposition. Mol. Cell. Biol. 10: 1017–1022.
Picologlou, S., M.E. Dicig, P. Kovarik & S. W. Liebman, 1988. The same configuration of Ty elements promotes different types and frequencies of rearrangements in different yeast strains. Mol. Gen. Genet. 211: 272–281.
Rolfe, M., A. Spanos & G. Banks, 1986. Induction of yeast Ty element transcription by ultraviolet light. Nature 319: 339–340.
Rose, M. & F. Winston, 1984. Identification of a Ty insertion within the coding sequence of the S. cerevisiae URA3 gene. Mol. Gen. Genet. 193: 557–560.
Rothstein, R., 1979. Deletions of a tyrosine tRNA gene in S. cerevisiae. Cell 17: 185–190.
Sandmeyer, S. B., L. J. Hansen & D. L. Chalker, 1990. Integration specificity of retrotransposons and retroviruses. Annu. Rev. Genet. 24: 491–518.
Sawyer, S. & D. L. Hartl, 1986. Distribution of transposable elements in prokaryotes. Theoret. Pop. Biol. 30: 1–16.
Sawyer, S. A., D. E. Dykhuizen, R. F. Dubose, L. Green, T. Mutangadura-Mhlanga, D. F. Wolczyk & D. L. Hartl, 1987. Distribution and abundance of insertion sequences among natural isolates of Escherichia coli. Genetics 115: 51–63.
Scherer, S., C. Mann & R. W. Davis, 1982. Reversion of a promoter delection in yeast. Nature 298: 815–819.
Shrimpton, A. E., T. F. C. Mackay & A. J. Leigh Brown, 1990. Transposable element-induced response to artificial selection in Drosophila melanogaster, molecular analysis of selected lines. Genetics 125: 803–811.
Simchen, G., F. Winston, C. A. Styles & G. R. Fink, 1984. Ty mediated gene expression of the LYS2 HIS4 genes of Saccharomyces cerevisiae is controlled by the same SPT genes. Proc. Natl. Acad. Sci. USA 81: 2431–2434.
Stahl, F. W., 1988. Bacterial genetics. A unicorn in the garden. Nature 335: 112–113.
Stavenhagen, J. B. & D. M. Robins, 1988. An ancient provirus has imposed androgen regulation on the adjacent mouse sex-limited protein gene. Cell 55: 247–254.
Stucka, R., H. Lochmuller & H. Feldmann, 1989. Ty4, a novel low-copy number element in Saccharomyces cerevisiae: one copy is located in a cluster of Ty elements and tRNA genes. Nuc. Acids Res. 17: 4993–5001.
Syvanen, M., 1984. The evolutionary implications of mobile genetic elements. Ann. Rev. Genet. 18: 271–293.
Taguchi, A. K. W., M. Ciriacy & E. T. Young, 1984. Carbon source dependence of transposable element-associated gene activation in Saccharomyces cerevisiae. Mol. Cell. Biol. 4: 61–68.
Toh-e, Y., Y. Kaneko, J. Akimaru & Y. Oshima, 1983. An insertion mutation associated with constitutive expression of repressible acid phosphatese in Saccharomyces cerevisiae. Mol. Gen. Genet. 191: 339–346.
Voelker, R. A., J. Graves, W. Gibson & M. Eisenberg, 1990. Mobile element insertions causing mutations in the Drosophila suppressor of sable locus occur in DNase I hypersensitive subregions of 5′-transcribed nontranslated sequences. Genetics 126: 1071–1982.
Warmington, J. R., R. B. Waring, C. S. Newlon, K. J. Indge & S. G. Oliver, 1985. Nucleotide sequence characterization of Ty 1–17, a class II transposon from yeast. Nuc. Acids Res. 13: 6679–6693.
Weinstock, K. G., M. F. Mastrangelo, T. J. Burkett, D. J. Garfinkel & J. N. Strathern, 1990. Multimeric arrays of the yeast retrotransposon Ty. Mol. Cell. Biol. 10: 2882–2892.
Wilke, C. M. & J. Adams, 1992. Fitness effects of Ty transposition in Saccharomyces cerevisiae. Genetics 131: 31–42.
Wilke, C. M., S. H. Heidler, N. Brown & S. W. Liebman, 1989. Analysis of yeast retrotransposon Ty insertions at the CAN1 locus. Genetics 123: 655–665.
Williamson, V. M., E. T. Young & M. Ciriacy, 1981. Transposable elements associated with constitutive expression of alcohol dehydrogenase II. Cell 23: 605–614.
Wilson, W., M. H. Malim, J. Mellor, A. J. Kingsman & S. M. Kingsman, 1986. Expression strategies of the yeast retrotransposon Ty: a short sequence directs ribosomal frameshifting. Nuc. Acids Res. 14: 7001–7016.
Xu, H. & J. D. Boeke, 1990. Host genes that influence transposition in yeast: the abundance of a rate tRNA regulates Ty1 transposition frequency. Proc. Natl. Acad. Sci. USA 87: 8360–8364.
Yougren, S. D., J. D. Boeke, N. J. Sanders & D. J. Garfinkel, 1988. Functional organization of the retrotransposon Ty from Saccharomyces cerevisiae: Ty protease is required for transposition. Mol. Cell. Biol. 8: 1421–1431.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wilke, C.M., Maimer, E. & Adams, J. The population biology and evolutionary significance of Ty elements in Saccharomyces cerevisiae . Genetica 86, 155–173 (1992). https://doi.org/10.1007/BF00133718
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00133718