Abstract
Conjugation of biomolecules over membrane surfaces intended for blood contacting applications has been proven worthwhile for the improvement of their hemocompatibility. In conjunction with this factuality, this manuscript for the first time unveils the feasibility of bovine serum albumin (BSA) immobilization via polydopamine precursors on electrospun poly(ethylene-co-vinyl alcohol) (EVAL) leukodepletion filter membranes. The successful immobilization of different extents of BSA on EVAL membranes was endorsed by the Attenuated Total Reflectance—Fourier Transform Infrared (ATR-FTIR) Spectrum, variations in the wettability, electron microscopic images revealing the alterations in the fiber diameter and porousness of membranes. An interesting backlash was encountered in the in vitro plasma protein adsorption and in vitro platelet adhesion on the BSA-immobilized membranes as they exhibited a higher protein adsorption and lower platelet adhesion in comparison with neat EVAL. The infiltration of immobilized BSA to the leukodepletion capacity of those membranes was preliminarily studied by in vitro blood-material interactions followed by typical whole blood filtration of selected membranes. The data presented highlight that BSA moieties do not particularly affect the white blood cell (WBC) adhesion and platelet adhesion of EVAL membranes whereas resulted in a significantly higher red blood cell (RBC) recovery and decreased hemolysis under in vitro conditions. Accordingly, BSA-immobilized EVAL membranes were made to asymmetric leukodepletion filters; however, their further evaluation by whole blood filtration ensued that immobilization of BSA could not particularly enhance the overall whole blood leukodepletion efficacy of EVAL membranes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Attuning a biomaterial or membrane surface towards a more blood-congenial interface for blood contacting applications is conventionally thought of as a challenging venture. However, after understanding the role of material chemistry in the mechanism of blood-material interactions, it was possible to tackle the phenomena happening at the blood-material interface in a manner favorable for the desired application. Thus recent progresses in the realm of surface modification of biomaterials hitherto have proposed diverse strategies for minimizing the incompatibilities when such materials are supposed to interact with blood or blood components implicitly or explicitly [1, 2]. Numerous approaches have been disclosed for the effective response of the interface in many facets of hemocompatibility including protein non-fouling surfaces, inhibition to platelet adhesion and activation, adequate blood clotting time, minimized inflammation, reduced hemolysis and so on [3, 4]. To summarize a few of the most efficacious routes of membrane modifications to bring in all or many of the above-mentioned aspects include introduction of polyethylene glycol (PEG) brushes, grafting of other monomers, incorporation of suitable additives like zwitterions, attaching different functionalities by coupling reactions, attaching biomolecules like proteins, anticoagulants including heparin, etc. [5,6,7,8]. Amidst these, decoration of membranes with coating or covalent attachment of proteins is not only a promising approach, but a bioinspired one over other synthetic materials.
It has been perceived that the serum protein, albumin, fend off the platelet adhesion and are extensively used for the amelioration of blood compatibility of biomaterials intended for hemodialysis and cardiovascular devices [9, 10]. This particular attribute can also be made beneficial for modifying leukodepletion filter membranes as most of such membranes have an undesirable platelet adhesion [11]. Subsequently, quite many surface modification routes like grafting of hydrophilic monomers, incorporation polyzwitterions, attachment of polyvinyl pyrrolidone, etc., have been taken into consideration to come up with excellent filter membranes having the capacity for selective leukocyte removal [12,13,14]. Herein we delineate an attempt for the betterment of hemocompatibility and leukodepletion efficiency of electrospun poly(ethylene-co-vinyl alcohol) (EVAL) membranes by covalent immobilization of bovine serum albumin (BSA) via polydopamine spacer. In spite of the competence in leukocyte removal, electrospun EVAL membranes disbenefit from high levels of platelet adhesion, which entails further contemplation [15]. Notwithstanding the fact that albumin is effective in repelling the platelets, membrane bound with albumin has not yet been investigated for their leukodepletion performance. Therefore, this study outlines the effect of BSA immobilization on the overall performance of electrospun EVAL leukodepletion filter membranes.
Although albumin can be physically adsorbed onto biomaterial surface, its reactive immobilization has gained most appreciation [16, 17]. There are quite lot intermediates having multiple reactive functionalities which tie up the bare biomaterial surface with and expedite the subsequent immobilization of albumin. Dopamine is the most accepted bonding material for protein immobilization although a few other intercessors such as poly(N-vinyl-2-pyrrolidone) (PVP) and poly(acrylic acid) have been recently proven worthwhile [18, 19]. The ease of its polymerization and formation of a stable overlay of polydopamine over any kind of membrane surface regardless of the physicochemical and morphological features of the membrane rendered dopamine and polydopamine as the excellent choice of precursors for protein immobilization.
To put it brief, electrospun EVAL membranes were immobilized with BSA to different extents via polydopamine precursor. The modified membranes were characterized for their physical, chemical and morphological features by ATR-FTIR spectroscopy, water contact angle (WCA) measurements, measurement of critical wetting surface tension (CWST) and scanning electron microscopy (SEM). Effect of the newly introduced BSA chains on the blood-material interactions as well as the efficiency of leukodepletion was also assessed.
2 Materials and methods
2.1 Materials
Poly (ethylene-co-vinyl alcohol) (EVAL) with 44 mol% ethylene content (having melting point 191 °C and melt index 3.50 g/10 min) was procured from Sigma-Aldrich, USA. Bovine serum albumin (BSA) and dopamine hydrochloride were obtained from Himedia, India. Acetone AR was procured from Rankem, India. 2-Propanol was purchased from Spectrochem, India. All the reagents were used as received.
2.2 Fabrication of EVAL membranes immobilized with of BSA via polydopamine spacer (EVAL-BSA)
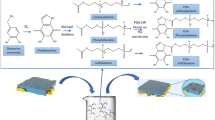
Neat and dried EVAL membranes, prepared by electrospinning of EVAL solution in 2-propanol/water mixture at a rate of 10 mL/h, were initially placed in 2 g/dL dopamine solution (10 mM in tris HCl) and incubated at 30 °C for 12 h. During the period, the dopamine polymerizes to polydopamine (PD) particles and gets firmly attached to the EVAL membranes. As a result, the membranes turned black. This is also depicted in Fig. 1. The PD coated EVAL membranes (EVAL- PD) thus obtained were thoroughly washed with distilled water by ultrasound sonication for 8-10 times, by replacing the water at each 30 min, for removing any unbound/loosely bound PD particles. Later the washed membranes were dried under vacuum at 50 °C overnight. BSA solutions were prepared at various concentrations 1, 2, 10, 50 and 100 g/dL by dissolving the desired amount of BSA crystals in PBS of pH 7.4. The dried membranes of EVAL-PD were immersed in the BSA solution taken in a rectangular glass slot and incubated at 30 °C for 24 h. The membranes were then taken out and washed again with distilled water by an ultrasound sonication to extract the unattached BSA. After washing, the obtained BSA-immobilized EVAL membranes (EVAL-BSA) were dried in a vacuum oven at room temperature for 3 days. The various BSA-immobilized EVAL membranes were denoted EVAL-BSA along with their corresponding BSA concentration.
2.3 Characterization of EVAL-PD and EVAL-BSA membranes
All the membranes were analyzed for their surface chemistry by ATR-FTIR recorded on Jasco, Model 6300 (Japan). The hydrophilicity of the membranes was estimated by measuring their WCA recorded on a goniometer equipped with a camera (Data Physics OCA 15 Plus, Germany). The wettability control of the membranes was estimated by measurement of critical wetting surface tension (CWST) using a series of aqueous solutions varying surface tensions. The series of solutions used and the method of measurement have been reported in our earlier publication [20]. The morphological features of membranes were well studied by SEM (Hitachi model S-240, Japan). From the SEM images, the average fiber diameter, pore diameter and percentage porosity of the membranes were measured making use of ImageJ software [21].
2.4 Analysis of blood-material interactions and leukodepletion efficiency
The behavior of BSA-immobilized EVAL membrane surface at the interface with blood was established by the in vitro hemocompatibility evaluation following the standard protocols according to ISO 10993—part 4 [22]. With reference to this, effect of BSA immobilization on the in vitro plasma protein adsorption, in vitro platelet adhesion, in vitro WBC adhesion, in vitro RBC adhesion and in vitro hemolysis was considered as per the procedures reported earlier [6]. Quantitative estimation of plasma protein adsorption by the membranes was estimated by Lowry’s assay [23]. The percentage change in platelet adhesion before and after BSA immobilization was found by exposing the membranes to platelet rich plasma (PRP) [22]. This is further substantiated by SEM images of the membrane surface after exposure to PRP. The preliminary inspection on the efficacy of leukodepletion was performed under in vitro conditions on the custom-made prototypic device. Whole blood (WB) added with anticoagulant CPD-A was allowed to pass through the neat and BSA-immobilized EVAL membranes. The percentage adhesion of RBC, WBC and platelets was calculated considering the initial and final counts of each using the formula mentioned below.
A hemolytic evaluation was also necessary to comment on the safety of BSA immobilization [22]. After this preliminary evaluation, one best EVAL-BSA membrane were developed to whole blood asymmetric leukodepletion filter device. For this, an additional BSA-immobilized EVAL membrane was prepared by the same protocol described but applying a different collector speed (1500 RPM). While designing an asymmetric filter, one layer of this was placed as the bottom layer, while the top 4 layers were those prepared at a collection speed of 500 RPM. Each of these membranes was cut into 5 cm disks, washed with distilled water, dried, and sterilized by ethylene oxide gas. They were then assembled into an in-house built poly(methyl methacrylate (PMMA) filter housing of 5 cm diameter and having an internal depth of 3 mm. These filters were initially primed with phosphate-buffered saline (PBS) of pH 7.4. About 20 ml of freshly collected, anticoagulated human blood collected in a blood bag was then allowed to pass through EVAL-BSA filter, by means of infusion tubes, spontaneously under gravity. The filtered blood was collected to sterilized centrifuge tubes. The % adhesion of WBC, RBC and platelets was calculated from their respective initial and final counts using the aforementioned equation. Another hemolytic evaluation was also carried out with the filtered blood. The speed of filtration was calculated from the time taken for filtration. After the filtration, the filter membrane assembly was detached from the filter housing and further analyzed by SEM and histological examinations. The methods of sample processing for these two examinations have been reported elsewhere [15]. All these tests under in vitro hemocompatibility evaluation and whole blood filtration were done using human blood collected from healthy voluntary donor with informed consent, and the studies have been approved by the Institutional Ethics Committee of Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCT/IEC/594/2014 dated 21/04/2014).
2.5 Statistical analysis
The entire quantitative data were reported as mean ± SD values. Th number of variables for each measurement is as follows. WCA—6 measurements, fiber/pore diameter—25 measurements, porosity—9 measurements, all biological evaluation including in vitro measurements and blood filtration—6 replicates. The measurement of CWST, being an empirical method, gave single values upon repeated measurements and hence that dataset has been excluded from analysis of variance. For all other data, statistical analysis was conducted using one-way ANOVA in Microsoft Excel. The results were considered as statistically significant wherever the p-values were less than 0.05.
3 Results
3.1 Fabrication and characterization of EVAL-BSA membranes
An illustration of the experimental protocol engendering the EVAL-polydopamine (EVAL-PD) and BSA-immobilized EVAL (EVAL-BSA) membranes is provided in Fig. 1. The PD formation on EVAL membranes was visualized by the black coloration to EVAL membranes. The ATR-FTIR spectra of neat EVAL, EVAL-PD and various EVAL-BSA membranes, viz. EVAL-BSA-1, EVAL-BSA-2, EVAL-BSA-10, EVAL-BSA-50 and EVAL-BSA-100, are compared in Fig. 2. It is vivid from the spectra that there was a new peak formation at 1604 cm−1 in the wake of C=O groups derived from PD. Subsequent to the BSA immobilization, this peak was shifted to higher wave number, 1655 cm−1 which derives from the C=O moieties in BSA. The SEM pictures in Fig. 3 give credence to the formation of PD particles on the EVAL fiber surface which was overturned after the immobilization of BSA. The smooth morphology of the fibers was retained, with a gradual increase in the fiber diameter with the increase in the BSA concentration (Table 1). The aftermath of BSA immobilization is well reflected in the porousness of membranes. The pore diameter of EVAL-PD membranes was not able to measure precisely due to dearth of well-defined and sharp boundaries as a repercussion to protrusive PD particles. Interestingly, the pore diameter was significantly reducing after BSA immobilization, while the percentage porosity was decreased initially after the formation of PD particles and further increased after the BSA immobilization (Table 1). Hence the percentage porosity of the neat EVAL and EVAL-BSA membranes remained more or less uniform. The fiber diameter, pore diameter and % porosity for the EVAL-BSA-10 membranes prepared at a collection speed of 1500 RPM, meant for constructing asymmetric leukodepletion filer, were respectively 2.1 ± 0.5 μm, 10.7 ± 2.6 μm and 52 ± 0.3%. The decrement in pore size with the increase in collection speed was significant (p < 0.05), Similarly, the WCA was found to be declining after the formation of PD layer, which points out to the enhanced hydrophilicity of EVAL membranes. However, the WCA was increased after the BSA immobilization (Table 1). So it has to be noted that the BSA chains offered hydrophobicity to the EVAL-PD membranes. The overall hydrophobicity of the EVAL-BSA membranes was near to that of the neat EVAL membranes. However, the wettability of the membranes was improved as a result of BSA immobilization as evident from a gradual increase in the CWST values.
3.2 Evaluation of the blood-material interaction and leukodepletion efficiency of EVAL-BSA interfaces
Table 2 shows the consequences of BSA immobilization on the various blood-material interactions. The plasma protein adsorption was found to be dramatically increasing with the increase in the immobilized BSA concentration. The adhesion of RBCs was gradually lessened, while the extent of WBC adhesion was kept unaltered. The platelet adhesion disported different trends when the both the neat and BSA-immobilized membranes were exposed to PRP and WB. No noticeable change in the platelet adhesion was observed when whole blood was filtered through the EVAL-BSA membranes, while the platelets from PRP became repellant to the EVAL-BSA membranes. This is also connoted from the SEM images of membrane exposed to PRP, provided in Fig. 4. Considerable extend of platelet adhesion was visualized on neat EVAL, while very few platelets were visible on all the EVAL-BSA membranes. The percentage hemolysis was also initially decreasing at lower concentrations of BSA, while the membranes, for which the BSA concentration was ≥ 50 g/L, induced a very high hemolysis.
From Table 2, it could be noticed that EVAL-BSA-10 is the ideal membrane having minimum hemolysis as well as decreased RBC and platelet adhesion. Hence, this membrane was devised to asymmetric filter and carried out the whole blood filtration studies. The efficiency was expressed in terms of % cell adhesion, % hemolysis as well as speed of filtration and is collated in Fig. 5. Upon whole blood filtration, it was observed that the WBC adhesion on the EVAL-BSA-10 membranes was reduced significantly and the RBC adhesion was enhanced than those of neat EVAL (Fig. 5a). No change in platelet adhesion was observed as a result of BSA immobilization. Interestingly, the speed of filtration was very much lower for the EVAL-BSA-10 filter than that of neat EVAL (Fig. 5c). The percentage hemolysis induced by the EVAL-BSA-10 filter was found to be approximately zero (Fig. 5b). Figures 6 and 7 depict the distribution of cells through the various layers of EVAL-BSA-10 asymmetric filter. From the microscopic images provided in Fig. 7, it can be visualized that all the layers were highly populated with cells. However, most of the WBCs were present in top and middle layers than bottom layer according to the SEM (Fig. 6) and microscopic images (Fig. 7). In addition, it can also be inferred that a higher number of RBCs (red stained) were present on EVAL-BSA-10 filter (Fig. 7) which is in good agreement with the quantitative data given in Fig. 5a.
4 Discussion
An attempt to covalently immobilize BSA onto electrospun EVAL membranes by means of pre-coating of polydopamine was executed in this article. The mechanism for this reaction has been well portrayed by profuse investigators [24, 25]. Dopamine is neurotransmitter which readily undergoes oxidative polymerization to form PD particles/aggregates under alkaline conditions [26]. An important feature of this PD is that it makes a very strong attachment to the substrate by covalent and non-covalent interactions like π–π interactions and electrostatic interactions [27]. Thus the newly generated PD layer remains stable and durable for a long time in all conditions excluding highly alkaline environment [27]. Thus the PD layer also furnishes its reactive functionalities for further attachment of desirable molecules and imparting the required properties to the substrate thereby. Consequently, lot many polymers have been functionalized by attachment of different molecules by means of adherent PD layer in order to enhance the wettability, cell adhesion and biocompatibility [28, 29].
The formation of PD and its further reaction with BSA on EVAL surface have been evidenced by structural, morphological, and wettability analysis. A new peak corresponding for the C=O stretch in PD has been featured in the ATR-FTIR spectra (Fig. 2). Many researchers have also noted this peak confirming the attachment of PD layer [30]. Further after the BSA immobilization, this peak was replaced by another peak which could be primarily due to the C=O stretch of Amide I band derived from BSA. Interestingly, no other peaks of Amide I band were visible at lower wavelength (Fig. 2). This alludes to the point that the NH2 groups of BSA were involved in the reaction with PD. Thus it is also concluded that BSA was not simply physically adsorbed to the PD-coated EVAL membrane, but covalently attached. Interestingly, the SEM images reveal the formation of PD particle aggregates on EVAL surface (Fig. 3b). Similar observation was also previously published by Jiang et al. [31]. However, the concentration of PD particles disappeared after reacting with BSA sustaining the smooth morphology of the EVAL fibers. The progressive increment in the fiber diameter after the reaction with BSA also supports to their successful immobilization (Table 1). A notable decrease in the pore diameter when the collection speed was increased from 500 RPM to 1500 RPM cues to the asymmetry in the leukodepletion filter. The effect of BSA immobilization on the wettability of EVAL membranes has also been studied. The results presented indicate that the EVAL membranes became slightly hydrophilic after the PD formation (Table 1). This is obviously due to the presence of hydrophilic catecholic groups derived from the PD [32]. However, the WCA values were raised after BSA immobilization. This stipulates that the BSA chains were hydrophobic in nature. Similar observation was previously noted by Weng et al. [33] when BSA was immobilized to anatase TiO2 film by means of pre-treated phosphoric acid. The measurement of CWST is believed to be rather significant than WCA of membranes, particularly for leukodepletion filters. An approximate CWST of 78 mN/m or higher is desirable for the blood to wet the filter membranes [13]. Although the BSA immobilization improved the CWST of EVAL, the maximum CWST noted as 76.3 mN/m. Prima facie, it might seem contrary that there is disagreement between WCA and CWST values for the various membranes. Nevertheless, CWST, an indirect measure of hydrophilicity, is enhanced by the decrease in WCA and CWST is also dependent on the surface coverage of the functional moieties and its surface polarity [34]. An increase in CSWT despite the hydrophobicity of the membranes could be attributed either to the presence of foldable polymeric chains/branches of polar character or to the extension of surface modification to the bulk of the polymer [34]. BSA being a globular protein having 585 amino acid residues prefers to take up a folded orientation and requires a liquid of still higher polarity/surface tension to wet its surface resulting in an increased CWST than neat EVAL.
The effects of BSA immobilization were clearly visible from the data of in vitro hemocompatibility evaluation. The in vitro platelet adhesion was effectively decreased when the EVAL-BSA membranes were exposed to PRP, which is in accordance with many other studies (Table 2, Fig. 4) [35, 36]. It was surprising to note that the plasma protein adsorption and percentage hemolysis was gradually increasing with the increase in the BSA concentration (Table 2). Previous studies have evidenced the existence of BSA as negatively charged species at the physiological pH due to an isoelectric point of 4.7 [37]. This points to the presence of ionizable –COOH groups taking dominancy over other functionalities. Thus, the increase in plasma protein may be ascribed to the active –COOH groups of the BSA, as they bring about an effective bonding between the various proteins in plasma. Moreover the BSA chains will also invite other proteins in the environment ultimately resulting in a high protein adsorption. In resemblance to this, a study Fu et al. [38] also demonstrated that –COOH groups encourages the protein adsorption as they observed EVAL membranes in situ functionalized with citric acid had significantly higher protein adsorption than the unmodified EVAL. Thus when whole blood was filtered through EVAL-BSA membranes both under in vitro conditions as well as a filter device, most of the proteins was adsorbed to its surface [38]. The effects due to this phenomenon are also reflected in other parameters also when whole blood was allowed to permeate through the EVAL-BSA membrane. An incursion by the better part of the platelets and WBCs upon the BSA membranes was noteworthy in both cases. However, the RBC adhesion was reduced, in the in vitro exposure to blood, that is to say, the BSA chains offered an improved RBC recovery, which is highly demanded for leukodepletion filters (Table 2). Such an enhanced platelet adhesion from whole blood is paradoxical to what observed from PRP. It has been well reviewed that the adhesion of blood components to materials is influenced by many parameters, one of which being the viscosity of blood [39]. Thus the extent of protein adsorption from a more viscous whole blood might be quite different from that encountered with diluted plasma. It might be the formation of a strong protein layer on the EVAL-BSA membrane surface which facilitated the fine adhesion of WBC and the subsequent recruitment of platelets and vice versa. Such interrelated adhesion of platelets and WBCs has also been reported to occur on membrane oxygenators and filter membranes [40, 41]. Even certain leukodepletion filter membranes developed from hydrogel interfaces having different charge densities have shown a charge biased WBC to platelet adhesion, nevertheless their excellent blood compatibility in vitro [42]. So as a result of these adhesions, it is expected that pores of the membranes might be clogged with cells and proteins. In such a case the RBC might find it difficult to pass through the membrane pores. This was also evinced by the application of a high suction while performing the leukodepletion studies by whole blood filtration under in vitro conditions. Obviously, the RBCs were squeezed through the pores resulting in a high degree of lysis (Table 2). Thus all-inclusive, it can be inferred that it is the surface charge balance of the membranes cued from the different extents of BSA which ultimately decided the potency of the membranes apropos leukodepletion. Higher extent of BSA immobilization on EVAL membranes is inappropriate and among the various EVAL-BSA membranes, EVAL-BSA-10, with negligible hemolysis, is being proposed worthwhile for the development of filters suitable for preparing RBC concentrate from whole blood.
However, some interesting deviation from the in vitro blood-material behavior was observed as a result of typical filtration of whole blood. Notwithstanding the several published reports on the betterment of in vitro hemocompatibility of BSA functionalized biomaterials, there is no previous data on the leukodepletion efficiency by such membranes. The WBC capturing by the BSA chains was marginally lower than neat EVAL membranes. The extent of platelet adhesion was intact, whereas a remarkable increase in the % of RBC adhesion was noted. The reason for this is quite obvious that EVAL-BSA surfaces show high protein adsorption. This triggers the faster transfer of platelet, also vivid from the high platelet adhesion and longer filtration time. Subsequently, there are chances of rapid pore plugging effect which hindered the deformation of RBCs, also supported by the microscopic images (Fig. 7). This is in well agreement with earlier reports that the acidic groups cause an increase in the bending elastic modulus of RBCs which ultimately results in the decrement in extend of RBC deformation [12]. This is well reflected in the poor RBC recovery. In an earlier study by Gerard et al. [43], the effect of RGD peptides immobilized on poly(butylene terephthalate) non-woven fabric (PBTNF) upon leukocyte removal was investigated by whole blood filtration tests. However, only one layer of this modified layer was used in assembly with 14 layers of commercial unmodified PBTNF [43]. They observed only infinitesimal increment in WBC adhesion, however, there was no notion on its wettability level and the adhesion of other components [43]. Excluding this, there is no precedent report on the effect of surface immobilized proteins on leukodepletion. There was a controversial disparity in the performance of the membranes under in vitro conditions and during filtration, because the adhesion of various components and the factors leading to them is entirely different under stationary (in vitro) and dynamic flowing conditions. It is noticeable that there are quite many investigations focusing on the surface modification of commercially available leukodepletion filter membranes and assessment of their efficiency only by in vitro blood-material interactions [44, 45]. Very few researchers delved into the utility of such membrane toward a typical device filtration. Even in those works, instead of whole blood various blood fragments like platelet concentrates, RBC concentrate etc. were used to assess the filter efficiency [12, 45]. The results of such investigations could not be compared with that of whole blood filtration, as is the case here, because the relative population of WBC will be quite different in both cases so do the capacity of the filter. Interestingly, in a report by Cao and co-workers where commercial grade PBTNF used in leukodepletion filter device was immobilized with PVP with the intention of selective leukocyte removal, further evaluation by whole blood filtration could not particularly alter the platelet recovery, although the method as proven successful under in vitro conditions [14, 46]. Thus, the obtained results demonstrate that the BSA functionalization could improve the hemocompatibility of the EVAL membranes in vitro, however, could not significantly enhance the overall leukodepletion efficiency during whole blood filtration. However, a fair decrease in the platelet adhesion when PRP was exposed to BSA-immobilized membranes suggests that these membranes will be particularly beneficial in the filtration of platelet concentrates.
5 Conclusions
This work epitomizes the covalent immobilization of BSA to electrospun EVAL membranes utilizing the reactive polydopamine coating for improving the leukodepletion efficiency. Chemical characterization of the various EVAL-BSA membranes using ATR-FTIR confirmed the successful immobilization. Consequentially, there observed a gradual increment in fiber diameter and decrement in pore diameter while preserving the wettability and percentage porosity of the membranes. Upon the evaluation of blood-membrane interactions, a fair increment in the plasma protein adsorption as well as disinclination to platelet adhesion in vitro was noticed as a function of extent BSA immobilization. Upon whole blood exposure to the functionalized membranes, a marked increase in RBC recovery without affecting the innate capacity of WBC adhesion of EVAL membranes connoted the desirability of the BSA immobilization toward better leukodepletion performance. Congruous with this, a significant reduction in the hemolysis at lower proportions of immobilized BSA chains and intact platelet adhesion forecloses the expedience of higher extents of BSA immobilization to membranes intended for leukodepletion. The typical whole blood filtration studies project that the immobilization of BSA was not as beneficial as expected and could not ensure the selective removal of WBCs.
References
Gorbet M, Sperling C, Maitz MF et al (2019) The blood compatibility challenge part 3: material associated activation of blood cascades and cells. Acta Biomater 94:25–32. https://doi.org/10.1016/j.actbio.2019.06.020
Jaffer IH, Weitz JI (2019) The blood compatibility challenge part 1: blood-contacting medical devices: The scope of the problem. Acta Biomater 94:2–10. https://doi.org/10.1016/j.actbio.2019.06.021
Brash JL, Horbett TA, Latour RA, Tengvall P (2019) The blood compatibility challenge part 2: protein adsorption phenomena governing blood reactivity. Acta Biomater 94:11–24. https://doi.org/10.1016/j.actbio.2019.06.022
Maitz MF, Martins MCL, Grabow N et al (2019) The blood compatibility challenge part 4: surface modification for hemocompatible materials: passive and active approaches to guide blood-material interactions. Acta Biomater 94:33–43. https://doi.org/10.1016/j.actbio.2019.06.019
Lv J, Jin J, Han Y, Jiang W (2019) Effect of end-grafted PEG conformation on the hemocompatibility of poly (styrene-b-(ethylene-co-butylene)-b-styrene). J Biomater Sci Polym Ed 30:1670–1685. https://doi.org/10.1080/09205063.2019.1657621
Mayuri PV, Bhatt A, Joseph R, Ramesh P (2016) Effect of photografting 2-hydroxyethyl acrylate on the hemocompatibility of electrospun poly (ethylene-co-vinyl alcohol) fibroporous mats. Mater Sci Eng C 60:19–29. https://doi.org/10.1016/j.msec.2015.11.004
Mayuri PV, Bhatt A, Ramesh P (2019) Sulfobetaine-functionalized electrospun poly(ethylene-co-vinyl alcohol) membranes for blood filtration. J Appl Polym Sci 136:47057. https://doi.org/10.1002/app.47057
Ji M, Chen X, Luo J, Wan Y (2019) Improved blood compatibility of polysulfone membrane by anticoagulant protein immobilization. Colloids Surf B 175:586–595. https://doi.org/10.1016/j.colsurfb.2018.12.026
Sperling C, Houska M, Brynda E et al (2006) In vitro hemocompatibility of albumin–heparin multilayer coatings on polyethersulfone prepared by the layer-by-layer technique. J Biomed Mater Res Part A Off J Soc Biomater Jpn Soc Biomater Aust Soc Biomater Korean Soc Biomater 76:681–689. https://doi.org/10.1002/jbm.a.30519
Qi P, Maitz MF, Huang N (2013) Surface modification of cardiovascular materials and implants. Surf Coat Technol 233:80–90. https://doi.org/10.1016/j.surfcoat.2013.02.008
Paunovic D, Van Der Meer P, Kjeldsen-Kragh J et al (2004) Multicenter evaluation of a whole-blood filter that saves platelets. Transfusion 44:1197–1203
Yang C, Cao Y, Sun K et al (2011) Functional groups grafted nonwoven fabrics for blood filtration—the effects of functional groups and wettability on the adhesion of leukocyte and platelet. Appl Surf Sci 257:2978–2983
Lewis AL, Freeman RNT, Redman RP et al (2003) Wettable phosphorylcholine-containing polymers useful in blood filtration. J Mater Sci Mater Med 14:39–45
Cao Y, Liu J, Zhong R et al (2012) Surface modification of PBT nonwoven fabrics used for blood filtration and their blood compatibility study. Artif Cells Blood Substit Biotechnol 40:317–325
Mayuri PV, Anugya B, Sabareeswaran A, Ramesh P (2016) A novel leukodepletion filter from electrospun poly (ethylene-vinyl alcohol) membranes and evaluation of its efficiency. Int J Polym Mater Polym Biomater 65:183–190. https://doi.org/10.1080/00914037.2015.1099101
Khan W, Kapoor M, Kumar N (2007) Covalent attachment of proteins to functionalized polypyrrole-coated metallic surfaces for improved biocompatibility. Acta Biomater 3:541–549. https://doi.org/10.1016/j.actbio.2007.01.006
Bousalem S, Benabderrahmane S, Sang YYC et al (2005) Covalent immobilization of human serum albumin onto reactive polypyrrole-coated polystyrene latex particles. J Mater Chem 15:3109–3116. https://doi.org/10.1039/B500982K
Timin AS, Solomonov AV, Musabirov II et al (2014) Immobilization of bovine serum albumin onto porous poly (vinylpyrrolidone)-modified silicas. Ind Eng Chem Res 53:13699–13710. https://doi.org/10.1021/ie501915f
Tu M-M, Xu J-J, Qiu Y-R (2019) Surface hemocompatible modification of polysulfone membrane via covalently grafting acrylic acid and sulfonated hydroxypropyl chitosan. RSC Adv 9:6254–6266. https://doi.org/10.1039/C8RA10573A
Mayuri PV, Bhatt A, Ramesh P (2020) Glycine integrated zwitterionic hemocompatible electrospun poly(ethylene-co-vinyl alcohol) membranes for leukodepletion. Biomed Phys Eng Express. https://doi.org/10.1088/2057-1976/abac8f
Ghasemi-Mobarakeh L, Semnani D, Morshed M (2007) A novel method for porosity measurement of various surface layers of nanofibers mat using image analysis for tissue engineering applications. J Appl Polym Sci 106:2536–2542. https://doi.org/10.1002/app.26949
Seyfert UT, Biehl V, Schenk J (2002) In vitro hemocompatibility testing of biomaterials according to the ISO 10993-4. Biomol Eng 19:91–96. https://doi.org/10.1016/S1389-0344(02)00015-1
Olson BJ, Markwell J (2007) Assays for determination of protein concentration. Curr Protocols Protein Sci 48:48:3.4. 1-3.4. 29. https://doi.org/10.1016/S1389-0344(02)00015-1
Ryu JH, Hong S, Lee H (2015) Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: a mini review. Acta Biomater 27:101–115. https://doi.org/10.1016/j.actbio.2015.08.043
Chan W (2019) Investigation of the chemical structure and formation mechanism of polydopamine from self-assembly of dopamine by liquid chromatography/mass spectrometry coupled with isotope-labelling techniques. Rapid Commun Mass Spectrom 33:429–436. https://doi.org/10.1002/rcm.8373
Vaneckova T, Bezdekova J, Han G et al (2020) Application of molecularly imprinted polymers as artificial receptors for imaging. Acta Biomater 101:444–458. https://doi.org/10.1016/j.actbio.2019.11.007
Das MK, Sarma A, Deka T (2019) Polydopamine-based simple and versatile surface modification of polymeric nano drug carriers. In: Pathak YV (ed) Surface modification of nanoparticles for targeted drug delivery. Springer International Publishing, Cham, pp 369–389
Guo J, Suma T, Richardson JJ, Ejima H (2019) Modular assembly of biomaterials using polyphenols as building blocks. ACS Biomater Sci Eng 5:5578–5596. https://doi.org/10.1021/acsbiomaterials.8b01507
Xie C (2019) Bio-inspired nanofunctionalisation of biomaterial surfaces: a review. Biosurface Biotribol 5:83–92. https://doi.org/10.1049/bsbt.2019.0009
Zhu L-P, Jiang J-H, Zhu B-K, Xu Y-Y (2011) Immobilization of bovine serum albumin onto porous polyethylene membranes using strongly attached polydopamine as a spacer. Colloids Surf B 86:111–118. https://doi.org/10.1016/j.colsurfb.2011.03.027
Jiang J, Zhu L, Zhu L et al (2011) Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir 27:14180–14187. https://doi.org/10.1021/la202877k
Lyu Q, Hsueh N, Chai CLL (2019) The chemistry of bioinspired catechol(amine)-based coatings. ACS Biomater Sci Eng 5:2708–2724. https://doi.org/10.1021/acsbiomaterials.9b00281
Weng YJ, Hou RX, Li GC et al (2008) Immobilization of bovine serum albumin on TiO2 film via chemisorption of H3PO4 interface and effects on platelets adhesion. Appl Surf Sci 254:2712–2719. https://doi.org/10.1016/j.apsusc.2007.10.107
De Lange PJ, Gebben B, Elfrink PJ (1994) XPS analysis of polypropylene grafted with acrylic monomers. Surf Interface Anal 22:502–506
Wei Q, Li B, Yi N et al (2011) Improving the blood compatibility of material surfaces via biomolecule-immobilized mussel-inspired coatings. J Biomed Mater Res, Part A 96A:38–45. https://doi.org/10.1002/jbm.a.32956
Zhang C, Jin J, Zhao J et al (2013) Functionalized polypropylene non-woven fabric membrane with bovine serum albumin and its hemocompatibility enhancement. Colloids Surf B 102:45–52. https://doi.org/10.1016/j.colsurfb.2012.08.007
Wu L, Jasinski J, Krishnan S (2012) Carboxybetaine, sulfobetaine, and cationic block copolymer coatings: a comparison of the surface properties and antibiofouling behavior. J Appl Polym Sci 124:2154–2170
Fu Q, Wang X, Si Y et al (2016) Scalable fabrication of electrospun nanofibrous membranes functionalized with citric acid for high-performance protein adsorption. ACS Appl Mater Interfaces 8:11819–11829. https://doi.org/10.1021/acsami.6b03107
Qiu Y, Myers DR, Lam WA (2019) The biophysics and mechanics of blood from a materials perspective. Nat Rev Mater 4:294–311. https://doi.org/10.1038/s41578-019-0099-y
Wilm J, Philipp A, Müller T et al (2018) Leukocyte adhesion as an indicator of oxygenator thrombosis during extracorporeal membrane oxygenation therapy? ASAIO J 64:24–30. https://doi.org/10.1097/MAT.0000000000000586
Lien C-C, Chen P-J, Venault A et al (2019) A zwitterionic interpenetrating network for improving the blood compatibility of polypropylene membranes applied to leukodepletion. J Membr Sci 584:148–160. https://doi.org/10.1016/j.memsci.2019.04.056
Chen Y-W, Venault A, Jhong J-F et al (2017) Developing blood leukocytes depletion membranes from the design of bio-inert PEGylated hydrogel interfaces with surface charge control. J Membr Sci 537:209–219. https://doi.org/10.1016/j.memsci.2017.05.031
Gérard E, Bessy E, Hénard G et al (2011) Surface modification of poly (butylene terephthalate) nonwoven by photochemistry and biofunctionalization with peptides for blood filtration. J Polym Sci Part A Polym Chem 49:5087–5099
Gérard E, Bessy E, Hénard G et al (2012) Surface modification of polypropylene nonwovens with LDV peptidomimetics and their application in the leukodepletion of blood products. J Biomed Mater Res B Appl Biomater 100:1513–1523
Bruil A, Oosterom HA, Steneker I et al (1993) Poly (ethyleneimine) modified filters for the removal of leukocytes from blood. J Biomed Mater Res 27:1253–1268
Qin H, Nie S, Cheng C et al (2014) Insights into the surface property and blood compatibility of polyethersulfone/polyvinylpyrrolidone composite membranes: toward high-performance hemodialyzer. Polym Adv Technol 25:851–860
Acknowledgements
The author Dr. Mayuri P.V. is indebted to INSPIRE Fellowship (Grant No: IF 110109) awarded by Department of Science and Technology, Government of India, for the financial support to carry out this work. The team also thank Dr. Sabareeswaran A., Division of Experimental Pathology, Department of Applied Biology Biomedical Technology Wing Sree Chitra Tirunal Institute for Medical Sciences and Technology Satelmond Palace Campus Trivandrum, India – 695012, for kindly doing the histopathological examination of filter membranes.
Author information
Authors and Affiliations
Contributions
MPV was involved in conception of idea, experimental design, carrying out measurements, interpretation of results, fund acquisition, and drafting/revising of manuscript. AB was involved in experimental assistance, interpretation of results, and revising of manuscript. RP was involved in conception of idea, supervision, experimental design, interpretation of results, and revising of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest related to authorship and publication of this study.
Ethical Statement
This study includes human samples. For the tests using human blood, samples were collected from healthy voluntary donor, after obtaining informed consent from them, and the studies have been approved by the Institutional Ethics Committee of Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCT/IEC/594/2014 dated 21/04/2014).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mayuri, P.V., Bhatt, A. & Parameswaran, R. Investigation of the potency of leukodepletion filter membranes immobilized with bovine serum albumin via polydopamine spacer. SN Appl. Sci. 2, 1738 (2020). https://doi.org/10.1007/s42452-020-03515-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03515-2