Abstract
Background
Rosuvastatin has been shown to provide effective treatment of dyslipidaemia in patients with end-stage renal disease (ESRD) undergoing haemodialysis, but data from controlled trials are very limited on the pharmacokinetics and pharmacodynamics of rosuvastatin in this population.
Objective
The aim of the present study was to better define the pharmacokinetic and pharmacodynamic profiles of repeated doses of rosuvastatin at a starting dose of 10 mg/day in a group of patients with ESRD.
Study Design
This was a single-centre, open-label study of rosuvastatin 10 mg daily, given over a 16-day treatment period in patients with ESRD undergoing chronic haemodialysis.
Setting
The study was carried out at a single site in the USA.
Patients
Patients aged 18–65 years with ESRD who had been on dialysis for ≥3 months were eligible for inclusion. Of 12 patients enrolled, 11 were included in the pharmacokinetic and pharmacodynamic analysis and all were included in the safety evaluation. The mean age of patients was 43.9 years (range 24–60 years). Five patients were Caucasian, six were black and one was Hispanic.
Intervention
Patients received an oral dose of rosuvastatin 10 mg once daily in the morning for 16 consecutive days.
Main Outcome Measure
The primary objective was to estimate the degree of rosuvastatin accumulation in plasma by measuring the area under the plasma concentration time curve (AUC) from time zero to 24 h following a single dose of rosuvastatin 10 mg on day 1, and the AUC at steady state on day 15.
Results
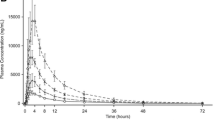
Following administration of single and multiple doses, plasma concentrations of rosuvastatin declined in an apparent bi-exponential manner and remained above the limit of assay detection throughout the entire sampling periods on both day 1 and day 15. Steady-state plasma concentrations of rosuvastatin were achieved by day 11. Little accumulation of rosuvastatin after repeated, once-daily dosing was observed; the geometric mean accumulation ratio for rosuvastatin was 1.37 (coefficient of variation = 36.4 %). Clearance of rosuvastatin and its metabolites via dialysis was minimal. Following rosuvastatin 10 mg daily for 16 days, total cholesterol, low-density lipoprotein cholesterol and apolipoprotein B were reduced from baseline by 30.6 %, 38.9 % and 30.6 %, respectively. Rosuvastatin was well tolerated.
Conclusion
The degree of rosuvastatin accumulation observed in patients receiving dialysis is similar to that in healthy individuals. The results of the current study suggest that rosuvastatin 10 mg may be administered to patients with ESRD on chronic haemodialysis without need for dose reduction.

Similar content being viewed by others
References
US Renal Data System, USRDS 2011 Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(Suppl. 3):S112–9.
Seliger SL, Weiss NS, Gillen DL, et al. HMG-CoA reductase inhibitors are associated with reduced mortality in ESRD patients. Kidney Int. 2002;61(1):297–304.
Mason NA, Bailie GR, Satayathum S, et al. HMG-coenzyme a reductase inhibitor use is associated with mortality reduction in haemodialysis patients. Am J Kidney Dis. 2005;45(1):119–26.
Wanner C, Krane V, März W, et al. German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238–48.
Fellström BC, Jardine AG, Schmieder RE, et al. AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360(14):1395–407.
Baigent C, Landray MJ, Reith C, et al. SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–92.
Holdaas H, Holme I, Schmieder RE, et al. Rosuvastatin in diabetic hemodialysis patients. J Am Soc Nephrol. 2011;22(7):1335–41.
März W, Genser B, Drechsler C, et al. German Diabetes and Dialysis Study Investigators. Atorvastatin and low density lipoprotein cholesterol in type 2 diabetes mellitus patients on hemodialysis. Clin J Am Soc Nephrol. 2011;6(6):1316–25.
Fellström BC, Holdaas H, Jardine AG, et al. Normalisation of CRP and LDL-C levels is related to reduction of cardiovascular morbidity and mortality in haemodialysis patients on rosuvastatin treatment—a post hoc analysis of the AURORA trial [abstract no. TH-PO473]. J Am Soc Nephrol. 2010;21:218A.
Nicholls SJ, Brandrup-Wognsen G, Palmer M, et al. Meta-analysis of comparative efficacy of increasing dose of Atorvastatin versus Rosuvastatin versus Simvastatin on lowering levels of atherogenic lipids (from VOYAGER). Am J Cardiol. 2010;105(1):69–76.
Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR trial). Am J Cardiol. 2003;92(2):152–60.
Martin PD, Warwick MJ, Dane AL, et al. Metabolism, excretion, and pharmacokinetics of rosuvastatin in healthy adult male volunteers. Clin Ther. 2003;25(11):2822–35.
Martin PD, Warwick MJ, Dane AL, et al. Absolute oral bioavailability of rosuvastatin in healthy white adult male volunteers. Clin Ther. 2003;25(10):2553–63.
Tzeng TB, Schneck DW, Birmingham BK, et al. Population pharmacokinetics of rosuvastatin: implications of renal impairment, race, and dyslipidaemia. Curr Med Res Opin. 2008;24(9):2575–85.
Crestor® Summary of Product Characteristics. http://www.medicines.org.uk/emc/document.aspx?documentid=11976. Accessed March 2012.
Crestor® (rosuvastatin calcium) Full Prescribing Information. AstraZeneca, Wilmington, Delaware 19850, USA. http://www1.astrazeneca-us.com/pi/crestor.pdf. Accessed March 2012.
Bologa R, Levine D, Parker T, et al. Pharmacokinetics of rosuvastatin in patients with end-stage kidney disease undergoing peritoneal dialysis. Clin Nephrol. 2009;72(6):437–41.
Martin PD, Mitchell PD, Schneck DW. Pharmacodynamic effects and pharmacokinetics of a new HMG-CoA reductase inhibitor, rosuvastatin, after morning or evening administration in healthy volunteers. Br J Clin Pharmacol. 2002;54(5):472–7.
Martin PD, Warwick MJ, Dane AL, et al. A double-blind, randomized, incomplete crossover trial to assess the dose proportionality of rosuvastatin in healthy volunteers. Clin Ther. 2003;25(8):2215–24.
Warwick MJ, Dane AL, Raza A, et al. Single- and multiple-dose pharmacokinetics and safety of the new HMG-CoA reductase inhibitor ZD4522 [abstract MoP19:W6]. Atherosclerosis. 2000;151(1):39.
Data on file:Domenic Sica MD (Principal Investigator). The pharmacokinetics and pharmacodynamics of ZD4522 (20 mg) in subjects with normal and impaired renal function. AstraZeneca Study 4522IL/0017, AstraZeneca, Wilmington, DE, USA; 2001.
Dreisbach AW. The influence of chronic renal failure on drug metabolism and transport. Clin Pharmacol Ther. 2009;86(5):553–6.
Leblond FA, Giroux L, Villeneuve JP, et al. Decreased in vivo metabolism of drugs in chronic renal failure. Drug Metab Dispos. 2000;28(11):1317–20.
Leblond F, Guévin C, Demers C, et al. Downregulation of hepatic cytochrome P450 in chronic renal failure. J Am Soc Nephrol. 2001;12(2):326–32.
Nolin TD, Frye RF, Sadr H, et al. The pharmacokinetics of fexofenadine but not midazolam are altered in end-stage renal disease. Clin Pharmacol Ther. 2008;83(Suppl. 1):S58.
Anzai N, Endou H. Renal drug transporters and nephrotoxicity. In: Proceedings of the 6th world congress on alternatives and animal use in the life sciences, August 21–25, 2007, Tokyo, Japan. AATEX 2007; 14 Suppl.: 447–52.
Dreisbach AW, Lertora JJ. The effect of chronic renal failure on drug metabolism and transport. Expert Opin Drug Metab Toxicol. 2008;4(8):1065–74.
Turpeinen M, Koivuviita N, Tolonen A, et al. Effect of renal impairment on the pharmacokinetics of bupropion and its metabolites. Br J Clin Pharmacol. 2007;64(2):165–73.
Vanholder R, Glorieux G, de Smet R, et al. New insights in uremic toxins. Kidney Int. 2003;63(Suppl. 84):S6–10.
Fuh MM, Lee CM, Jeng CY, et al. Effect of chronic renal failure on high-density lipoprotein kinetics. Kidney Int. 1990;37(5):1295–300.
Alabakovska SB, Todorova BB, Labudovic DD, et al. LDL and HDL subclass distribution in patients with end-stage renal diseases. Clin Biochem. 2002;35(3):211–6.
Muntner P, Coresh J, Smith JC, et al. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int. 2000;58(1):293–301.
Janicki K, Solski J, Janicka L, et al. Lipid and apolipoproteins (ApoAI, ApoB, Apo CIII, ApoE) disturbance in hemodialysis (HD) and renal transplant (Tx) patients. Ann Univ Mariae Curie Sklodowska Med. 2004;59(1):459–66.
Jamoussi K, Ayedi F, Abida N, et al. Lipid profile in maintenance haemodialysis [in French]. Pathol Biol (Paris). 2005;53(4):217–20.
Jones PH, Hunninghake DB, Ferdinand KC, et al. Effects of rosuvastatin versus atorvastatin, simvastatin, and pravastatin on non-high-density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia: additional results from the STELLAR trial. Clin Ther. 2004;26(9):1388–99.
LaRosa JC, Grundy SM, Waters DD, et al. Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352(14):1425–35.
Kidney Disease Outcomes Quality Initiative (K/DOQI) Group. K/DOQI clinical practice guidelines for managing dyslipidemias in chronic kidney disease. Am J Kidney Dis. 2003;41(Suppl. 3):S1–92.
Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44(3):720–32.
Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81.
Ridker PM, MacFadyen J, Cressman M, et al. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention-an Intervention Trial Evaluating Rosuvastatin) trial. J Am Coll Cardiol. 2010;55(12):1266–73.
Acknowledgments
This study was sponsored by AstraZeneca. The sponsor was involved in the design of the study and the collection, analysis and interpretation of the data, as well as the writing of the report, and was involved in the decision to submit the paper for publication. PM and CA are employees of AstraZeneca. BB, YW, TP and JZ are former employees of AstraZeneca. BB is currently employed by Yoh, a Service Provider to AstraZeneca Pharmaceuticals (Philadelphia, PA, USA), YW is currently employed by Purdue Pharma (Stamford, CT, USA), TP is currently employed by Janssen Pharmaceutical (Springhouse, PA, USA), JZ is currently employed by Amgen, PKDM-Seattle (Seattle, WA, USA). SS is a former employee of DaVita Clinical Research & Hennepin County Medical Center, and is currently employed by PAREXEL (Billerica, MA, USA). BB, PM, CA, JZ and YW all hold stock in AstraZeneca. Dennis Schneck, a former employee of AstraZeneca, acted as a medical advisor and was involved in the study design. Medical writing support was provided by Neil Venn and Kerry Knight of Prime Medica Ltd (Knutsford, Cheshire, UK) and editorial support by Maren White of White Quill Ltd, supported by AstraZeneca. Responsibility for opinions, conclusions, and interpretation of data lies with the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Birmingham, B.K., Swan, S.K., Puchalski, T. et al. Pharmacokinetic and Pharmacodynamic Profile of Rosuvastatin in Patients with End-Stage Renal Disease on Chronic Haemodialysis. Clin Drug Investig 33, 233–241 (2013). https://doi.org/10.1007/s40261-013-0071-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-013-0071-3