Abstract
Introduction
Coronary angiography (CAG) is the standard modality for assessment of coronary stenoses and intraprocedural guidance of percutaneous coronary interventions (PCI). However, the limitations of CAG are well recognized. Intracoronary imaging (ICI) can potentially overcome these limitations. Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) are the main ICI techniques utilized in clinical practice.
Aim
This narrative literature review addresses the current clinical applications of OCT in relation to IVUS and CAG in patients with coronary artery disease (CAD). Items reviewed are: technical implications of OCT and IVUS, lesion characterization and decision-making, stent optimization criteria, post-stenting results, safety in terms of procedural complications, clinical outcomes, and indications.
Main Findings
OCT is able to reveal more detail than IVUS due to its higher resolution. However, this higher resolution comes at the cost of a lower penetration depth. Pre-stenting OCT results in procedural change in more than 50% of the cases in terms of stent length and diameter. Post-stenting OCT resulting in stent optimization is reported in at least 27% of the cases. Malapposition and under-expansion are treated with post-dilatations, while edge dissections are treated with additional stent placement. Stent expansion, stent apposition, distal stent edge dissections, and reference lumen areas seem to be the most important stent optimization criteria for both decision-making and for reducing the risk of adverse events during follow-up. Both OCT and IVUS are superior in terms of post-stenting results compared with CAG alone. However, there is no consensus about whether OCT guidance results in better stent expansion than IVUS guidance. OCT, IVUS, and CAG are safe procedures with few reported procedural complications. In general, OCT guidance seems to contribute to favorable clinical outcomes compared with CAG guidance only. However, OCT guidance results in similar clinical outcomes as with IVUS guidance. OCT could be considered for lumen assessment and stent-related morphology in more complex cases in which CAG interpretation remains uncertain. Since OCT and IVUS have distinct characteristics, these techniques are complementary and should be considered carefully for each patient case based on the benefits and limitations of both techniques.
Similar content being viewed by others
Why carry out this study? |
Despite growing evidence, the adoption of coronary optical coherence tomography (OCT) in clinical practice remains limited. |
This study aimed to assess the current clinical applications of OCT in relation to intravascular ultrasound (IVUS) and coronary angiography (CAG) in patients with coronary artery disease. |
What was learned from the study? |
Both OCT and IVUS are superior in terms of post-stenting results compared with CAG alone. |
OCT and IVUS are complementary and should be considered carefully for each patient, based on the benefits and limitation of both techniques. |
Further research is needed to assess which patients benefit the most from intracoronary imaging, ideally differentiating between OCT and IVUS. |
Introduction
Coronary angiography (CAG) is the standard modality for the assessment of coronary stenoses and intraprocedural guidance of percutaneous coronary interventions (PCI) [1]. However, CAG has some well-recognized limitations. CAG results in a two-dimensional luminogram of a complex three-dimensional structure, which mainly shows luminal dimensions. CAG is limited in characterization of tissue or plaque (except for calcium, coarse ulcerations, or large dissections) and assessing features associated with suboptimal stent deployment [1,2,3,4]. However, these characteristics all contain important prognostic information, necessitating more advanced visualization.
Intracoronary imaging (ICI) can potentially overcome these limitations of CAG. According to the recent guidelines on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS), ICI can potentially be used during the diagnostic process of the evaluation of stenosis severity, lesion morphology, and the characterization of plaque composition [5]. The guideline states that ICI should be considered for (1) optimizing stent implantation and (2) detecting stent-related mechanical problems leading to restenosis.
Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) are the most common techniques for ICI and provide cross-sectional images of the vessel wall with a high resolution [6, 7]. The benefits of IVUS guidance on clinical outcome and stent optimization have been reported in multiple meta-analyses [8,9,10,11,12]. However, the benefits of OCT in relation to IVUS are not always clear. Despite growing evidence, the adoption of ICI in clinical practice remains limited [13].
This narrative literature review aimed to assess current clinical applications of OCT, in relation to IVUS and CAG in patients with coronary artery disease (CAD). This review addresses:
-
(1)
A short comparison of technical implications of OCT and IVUS;
-
(2)
Lesion characterization and decision-making;
-
(3)
Stent optimization criteria;
-
(4)
Post-stenting results;
-
(5)
Safety in terms of procedural complications;
-
(6)
Clinical outcomes;
-
(7)
Indications.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Technological Implications
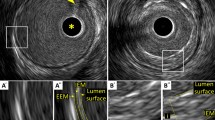
OCT is often defined as analogous to IVUS, as both techniques result in cross-sectional images by measuring the echo time delay and magnitudes of backscattered waves. However, the optical aspect of OCT compared to ultrasound has certain implications. First, OCT provides an axial spatial resolution of 10 to 20 µm whereas IVUS provides an axial spatial resolution of 100 to 200 µm. Lateral resolutions are typically 20 µm and 200 µm for OCT and IVUS, respectively. In contrast, IVUS has a maximum penetration depth of 10 mm, where OCT has a penetration depth of only 1 to 2.5 mm [14,15,16,17]. OCT is able to reveal more detail than IVUS due to its higher resolution. However, image interpretation should be performed carefully, as it is not clear whether small detailed abnormalities are clinically relevant [18].
With the introduction of Fourier domain OCT, high image acquisition speeds can be acquired with OCT (up to 25 mm/s). A major drawback of OCT imaging is the need for a contrast agent for blood clearance, as near-infrared light is fully attenuated by blood. Especially for patients with renal dysfunction, this extra use of contrast increases the risk of contrast-induced nephropathy (CIN) [15]. Another drawback of OCT is the inability to image ostial lesions as blood clearance is hampered, if not impossible [16, 19]. The technical specifications of OCT and IVUS are summarized in Table 1.
Lesion Characterization and Decision-Making
Plaque rupture is one of the main causes of myocardial infarction (MI). The most vulnerable plaques are those with a large lipid core and a thin fibrous cap [20, 21]. A thin fibrous cap (< 65 µm), large lipid core (lipid in ≥ 2 quadrants in any image), and activated macrophages (multiple punctate signal-rich regions) near the fibrous cap were identified as characteristics of vulnerable plaques in OCT autopsy studies [22,23,24,25,26]. Future clinical trials should demonstrate whether OCT can definitively distinguish vulnerable from stable plaques. Recently, spontaneous coronary artery dissection (SCAD) gained recognition as a cause of acute coronary syndrome (ACS), specifically in women. The exact pathophysiology of SCAD remains fairly unknown. Nonetheless, ICI can help to identify the false lumen and intramural hematoma between the intima and media resulting in vessel occlusion, as it is difficult to distinguish SCAD from atherosclerotic lesions with conventional CAG [27]. OCT studies suggest the presence of a crescent-shaped false lumen and the presence of fenestrations between the true lumen and false lumen as characteristics of SCAD [28,29,30,31]. Most experts recommend OCT over IVUS to assess SCAD, due to its better spatial resolution [32]. Furthermore, ICI provides insight into the composition of coronary arterial thrombus and stent thrombosis. OCT seems especially suitable for assessing thrombus, due to the higher resolution and the attenuation of the OCT signal by red blood cells [33, 34]. Thrombus is characterized as an irregular mass (≥ 250 µm) protruding into the lumen [33, 35]. Red thrombus (erythrocyte-rich) is visualized by OCT as a high-backscattering projection with signal-free shadowing. White thrombus (platelet-rich) is visualized as a signal-rich, low-backscattering projection [33].
Pre-stenting OCT results in procedural change in 57–71.4% of the lesions. Most changes include changes in stent length and diameter [36,37,38]. Wijns et al. [36] found no change in the number of stents implanted [36]. Leistner et al. [37] found that changes in strategy occurred more frequently in complex lesions (60.7% in complex lesions vs. 10.7% in simple lesions, p = 0.01) [37]. Remarkably, Meneveau et al. [38] reported no differences in stent length or diameter. However, Meneveau et al. reported an increase of glycoprotein inhibitors as OCT was able to visualize a significant higher rate of thrombus [38]. Differences in outcome can be explained by the different patient characteristics between the studies. More than 80% of the patients in the study by Leistner et al. are characterized as stable CAD. Meneveau et al. only included patients with non-ST elevation MI (NSTEMI), whereas Wijns et al. included patients with stable CAD, unstable CAD, and NSTEMI.
Multiple studies report on the effect of post-stenting OCT on decision-making [36,37,38,39,40]. Stent optimization was performed in 27–52.2% of the lesions. Decisions were mainly based on stent malapposition, under-expansion, or edge dissections. Malapposition and under-expansion resulted in post-dilatation, while edge dissections resulted in additional stent placement. Meneveau et al. [38] reported post-stenting optimization based on OCT in 50% of the patients compared with 22.5% in the CAG group (p < 0.0001) [38].
Stent Optimization Criteria
IVUS or OCT should be considered for stent optimization, according to recent guidelines and consensus documents [5, 13, 41, 42]. Most studies included in this review used stent optimization criteria derived from the MUSIC study by De Jaegere et al. [43]. De Jaegere et al. were the first to establish criteria for optimal stent expansion by IVUS guidance. Under-expansion, malapposition, and edge dissections are the most important optimization criteria. Although small variations occur, under-expansion was mostly defined by a minimal stent area (MSA) or minimal lumen area (MLA) < 90% of the average reference lumen area [2, 37,38,39,40, 44,45,46,47]. Malapposition was defined as a stent lumen distance > 200 µm. Malapposition was indicated for optimization when present in at least five consecutive frames or in three consecutive struts [36, 37, 39, 45, 48, 49]. Edge dissection was defined as a linear rim of tissue ≥ 200 µm, 5 mm proximal or distal from the stent edge. Edge dissections were optimized when present in more than five consecutive frames [36, 39, 40, 45, 49]. Most studies used more optimization criteria, such as the presence of thrombus, tissue protrusions, and complete lesion coverage. However, these criteria differed considerably between studies. Full criteria used by the OCT studies are provided in supplementary Table S1.
Post-Stenting Results
Numerous studies have demonstrated that IVUS guidance compared with CAG guidance results in larger luminal dimensions and thus reduce the incidence of major adverse cardiovascular events (MACE) during follow-up. Stent expansion after PCI is the most compelling predictor of early stent thrombosis and restenosis [2, 8, 9, 12, 50,51,52,53]. However, Wijns et al. [36] reported a decrease in stent diameter in 31% of the lesions based on pre-stenting OCT [36]. According to a randomized controlled trial (RCT) by Habara et al. [44] comparing OCT guidance with IVUS guidance in 70 patients, OCT guidance was associated with a smaller stent expansion compared with IVUS guidance. Nonetheless, strong correlations were found between MSA and mean stent area comparing OCT with IVUS (r = 0.96 and r = 0.95, respectively, p < 0.0001). Habara et al. mentioned the low penetration depth of OCT as a potential factor driving under-expansion [44].
Prati et al. retrospectively analyzed end-procedural OCT findings and the risk of MACE in 832 patients. MACE was defined as a composite of all-cause mortality, myocardial infarction (MI), and target lesion revascularization (TLR). In-stent MLA < 4.5 mm2 (HR 1.64 (1.1–2.6), p = 0.040), distal dissection > 200 µm (2.54 (1.3–4.8), p = 0.004), distal reference lumen area < 4.5 mm2 (HR 4.65 (2.5–8.8), p < 0.001) and proximal reference lumen area < 4.5 mm2 (HR 5.73 (2.2–14.6), p < 0.001) were independent predictors of MACE. The absence of at least one significant criterion for optimal OCT stent deployment was also an independent predictor of MACE (HR 3.53 (2.2–5.8), p < 0.001) [49].
An RCT by Ali et al. [2] found that OCT guidance was non-inferior to IVUS guidance in terms of MSA. However, OCT was not superior. Compared to CAG guidance, OCT resulted in significant higher minimum and mean stent expansions (p = 0.02 and p = 0.001, respectively). No significant differences in MSA were found between OCT, IVUS, and CAG [2]. Maehara et al. [18] found similar results as Ali et al. [18, 36, 54]. However, malapposition, tissue prolapse, and edge dissections were detected more often with OCT than with IVUS.
Since the introduction of drug-eluting stents (DES), the rate of in-stent restenosis has declined [55]. Incomplete endothelial strut coverage is a predictor of late in-stent restenosis [56, 57]. Antonsen et al. (OCTACS, 2015) conducted an RCT on whether OCT-guided stenting resulted in improved stent strut coverage at 6 months compared with CAG guidance only. In total, 85 patients were included in a single center in Denmark. The percentage of uncovered struts was significantly lower in the OCT group (4.3% (1.2–9.8) vs. 9.0% (5.5–14.5), p < 0.01). In addition, OCT guidance led to completely covered struts in 17.5% of the cases vs. 2.2% for CAG guidance (p = 0.02). OCT guidance led to a significant reduction in the total malapposition area, volume, and the percentage of malapposed struts directly after stenting. However, no differences were observed in stent malapposition or MSA after 6 months [45]. An RCT by Lee et al. [58] found similar results [58]. An RCT by Meneveau et al. [38] compared post-procedural fractional flow reserve (FFR) after OCT guidance and CAG guidance. OCT guidance was performed both before and after stent placement. Multiple OCT runs could be performed until satisfactory results were acquired. As a result, FFR values of the OCT group were significantly higher (0.94 ± 0.04 vs. 0.92 ± 0.05, p = 0.005). In addition, the number of patients with an FFR > 0.90 at the end of the procedure was significantly higher in the OCT group (82.5 vs. 64.2%, p = 0.0001) [38]. Gatto et al. [59] retrospectively analyzed 125 lesions in patients who experienced MACE during 1-year follow-up. Fifty-seven lesions (54%) of 105 optimal CAG results showed suboptimal stenting results on OCT. Stent MLA < 4.5 mm2 and narrowing of the references were the most common features of suboptimal stent deployment identified with OCT [59].
Thus, OCT-guided stenting improves strut coverage and stent apposition, while reducing tissue protrusions compared to CAG guidance. OCT is non-inferior, but not superior to IVUS guidance. However, there is no consensus about whether OCT guidance or IVUS guidance results in better stent expansion. Full results of post-stenting results are provided in supplementary Table S2.
Procedural Complications
To establish whether OCT-guided PCI is a safe procedure, studies that addressed safety in the form of procedural complications after OCT-guided PCI were identified [2, 36, 38,39,40, 47, 60]. In general, the incidences of procedural complications, including contrast-induced nephropathy (CIN), were low and not different from either IVUS-guided or CAG-guided stenting. One propensity-matched cohort (1134 pairs) by Jones et al. [60] found that OCT-guided stenting was associated with even lower in-hospital MACE compared with CAG guidance alone (0.80 vs. 2.00%, p = 0.01) [60]. Full results of procedural complications are provided in supplementary Table S3.
Clinical Outcomes
To establish whether OCT-guided stenting results in favorable clinical outcomes, studies which addressed clinical outcomes after OCT-guided stenting were identified [36, 38,39,40, 45, 47, 60, 61]. Although the evidence is scarce and follow-up times are short, clinical outcomes in terms of MACE seem favorable for OCT-guided stenting over CAG-guided stenting. However, no differences were observed between OCT-guided and IVUS-guided stenting. Full results are discussed below and an overview is provided in supplementary Table S4.
Imola et al. (2010) prospectively performed pre-PCI OCT in 40 patients with ambiguous lesions. Post-PCI OCT was performed in 74 patients for post-stent assessment. Clinical follow-up was available in 88 patients with mean follow-up of 4.6 ± 3.2 months. No deaths, MI, or stent thrombosis were observed. Angina recurrence was observed in three patients with restenosis, leading to one coronary artery bypass grafting (CABG) and one re-PCI [39].
Prati et al. (CLI-OPCI, 2012) retrospectively compared 335 matched patient pairs undergoing either OCT guidance plus CAG guidance or CAG guidance only. Twelve-month follow-up showed a significantly lower risk of cardiac death for the OCT group (4 (1.2%) vs. 15 (4.5%), p = 0.010). MI occurred in 18 (5.4%) vs. 29 (8.7%) patients in the OCT group and CAG group, respectively (p = 0.096). The incidence of the composite of cardiac death and MI was significantly lower in the OCT group (P = 0.006). After multivariable logistic regression analysis, propensity score-adjusted analysis and Cox proportional hazard analysis OCT remained associated with a significantly lower risk of cardiac death or MI. No differences were observed in stent thrombosis or TLR [40].
An RCT by Antonsen et al. (OCTACS, 2015) compared OCT-guided PCI with CAG-guided PCI in 85 patients with NSTEMI. During a 6-month follow-up, two patients (4%) from the CAG group had MACE (one subacute stent thrombosis and one cardiac death). No cardiac events were reported in the OCT group [45].
Wijns et al. (ILUMIEN I, 2015) reported on the occurrence of cardiac events in patients with unstable or stable angina or NSTEMI in a large intercontinental prospective trial. The data were analyzed based on four optimization groups: PCI without optimization based on OCT (N = 137), optimization based on pre-PCI OCT only (N = 163), optimization based on post-PCI OCT only (N = 40), and optimization based on both pre-PCI and post-PCI OCT (N = 65). In general, all rates of cardiac events were low. Device-oriented MACE after 30-day follow-up were observed in 8.8, 8, 12.5, and 1.5%, respectively. Patient-oriented MACE after 30-day follow-up were observed in 10.9, 9.8, 12.5, and 1.5%, respectively. Rates of periprocedural MI after 30-day follow-up were significantly lower when procedural changes were made based on pre-PCI and post-PCI OCT (p = 0.029). Other events, such as revascularization and stent thrombosis, rarely occurred [36].
An RCT by Meneveau et al. (DOCTORS, 2016) including 240 patients with non-ST elevation ACS (NSTE-ACS) reported similar rates of MACE for OCT optimization vs. CAG optimization groups after 6-month follow-up. There was one death in the OCT-guided group and one recurrent MI in each group. No stent thrombosis was observed and no significant difference in the rate of target vessel revascularization (TVR) [38].
Iannaccone et al. (FORMIDABLE, 2017) retrospectively analyzed 270 propensity matched patient pairs with ACS, comparing OCT-guided PCI with CAG-guided PCI. After a mean follow-up of 700 days, no differences in MI (6 vs. 6%, p = 0.86) were observed. MACE (11 vs. 16%, p = 0.06), TVR (2 vs. 4%, p = 0.15) and stent thrombosis (0 vs. 2.7%, p = 0.26) were numerically lower for OCT, but not significant [61].
An RCT by Kubo et al. (OPINION, 2017) aimed to demonstrate non-inferiority of OCT-guided PCI compared with IVUS guidance in terms of clinical outcome. The primary outcome was target vessel failure (TVF), defined as a composite of cardiac death, target-vessel-related MI and ischemia-driven TVR. Secondary outcomes were cardiac death, MI, vessel revascularization, lesion revascularization, MACE, stent thrombosis, restenosis, stroke, and CIN. In total, 791 patients were analyzed in a per-protocol analysis. Within 12 months, TVF was observed in 21 patients (5.2%) in the OCT-guided group vs. 19 patients (4.9%) in the IVUS-guided group, p = 0.042 for non-inferiority testing. No differences in secondary outcomes were observed. Most noteworthy, no cases of CIN occurred in both groups, although OCT led to a significant higher amount of contrast used during PCI (164 ± 66 ml vs. 138 ± 56 ml) [47].
Jones et al. (Pan-London PCI registry, 2018) analyzed the occurrence of all-cause mortality in a cohort of 87,166 patients who received PCI between 2005 and 2015. OCT was used in 1149 patients, IVUS in 10,971 patients, and CAG alone in 75,046 patients. A significant difference in mortality was found after a median follow-up of 4.8 years: 7.7% vs. 12.2% vs. 15.7% (p < 0.0001), respectively. This difference was observed for both elective as ACS subgroups. This difference persisted for OCT vs. CAG after multivariate Cox analysis and propensity score matching. No differences were found between matched OCT and IVUS cohorts [60].
Currently, two large RCTs are initiated to demonstrate the superiority of OCT-guided stent implantation compared to CAG-guided stenting in terms of MACE after 2 years of follow-up. The ILUMIEN IV trial is a multi-center RCT in 125 countries across the globe [62]. They aim to include 3656 patients. The first results are expected mid-2021, while the estimated completion date is mid-2022. The OCTOBER trial is a European RCT that aims to demonstrate the superiority of OCT-guided stenting in bifurcations lesions [63]. They aim to include 1200 patients. The first results are expected in May of 2021.
Indications
In a web-based survey among 1105 interventional cardiologists, stent optimization (88.5%), preprocedural strategy guidance (79.6%), and left-main interventions (77.0%) were the main indications for ICI. High costs (65.9%) and prolongation of the procedure (35.0%) were mentioned as the main factors limiting the use of ICI [13].
A recent consensus document by the European Association of Percutaneous Cardiovascular Interventions (EAPCI) appraised current evidence on clinical indications for ICI [42]. Pre-PCI strategy guidance and stent optimization are the main clinical applications of both OCT and IVUS [5, 13]. Patients with ACS and complex lesions, including left-main, bifurcation, chronic total occlusion (CTO) and long lesions, benefit the most from ICI regarding all-cause mortality and MACE [12, 50]. Two RCTs showed that OCT is non-inferior to IVUS regarding post-optimization results and clinical outcomes [2, 47]. The expert consensus group stated that IVUS and OCT are equivalent and both superior to CAG guidance. However, an extensive RCT that addresses superiority of OCT guidance in terms of clinical outcome is currently still missing. Therefore, the benefits and limitations of both techniques as mentioned earlier (see Technological Implications) should be considered carefully when selecting patients.
OCT has a limited penetration depth, especially in lipid-rich plaques. In contrast, calcified plaques can be visualized well with OCT, whereas IVUS is not capable of penetrating calcified plaques. Therefore, IVUS should be preferred for assessing plaque burden and vessel size in patients presenting with lipid-rich plaques, whereas OCT should be preferred for assessing calcified plaques. This is especially relevant in a research setting, as in clinical practice you might not know what type of plaque is present before assessment with ICI. In clinical practice, IVUS is mostly indicated for assessing ostial lesions of the left-main. OCT is not suitable in left-main lesions, due to the need for blood clearance. In addition, IVUS can be considered in patients presenting with CTO lesions after opening the vessel, as blood clearance by contrast injections may be challenging in these patients. In patients with renal dysfunction, IVUS is recommended, as no contrast injections are required. OCT has a much higher resolution compared with IVUS and should therefore be considered for lumen assessment and stent-related morphology, such as thrombosis, restenosis, edge dissections, expansion, and malapposition [42]. Interpretation of small abnormalities should be considered carefully, as the clinical impact of such abnormalities is unknown [18].
Prati et al. (2010) mentioned that CAG for suspected CAD results in normal angiograms in approximately 10 to 15% of the patients [16]. Yamamoto et al. (2019) found abnormal OCT findings in approximately 25% of the patients presenting insignificant lesions by CAG [64]. IVUS and OCT can both confirm the findings by CAG or indicate the subclinical lesion formation, resulting in an optimal therapeutic strategy for primary prevention. In general, ICI should be considered for the evaluation of intermediate stenoses and ambiguous lesions. Especially in cases of uncertain severity, very short lesions, pre-aneurysmal or post-aneurysmal lesions, ostial or left-main stenoses, branching sites, sites with focal spasm or angiographically hazy lesions. OCT should not be performed in cases where the expected plaque thickness exceeds the penetration depth [16].
Discussion
With this review, the clinical applications of OCT in patients with CAD were identified. OCT stenting optimization criteria were described. Both pre-PCI as post-PCI OCT affected physician decision-making. Pre-PCI OCT mainly affected the choice of stent length and stent diameter in patients with complex lesions. Post-PCI OCT resulted in post-procedural changes in 27% to 52.2% of the lesions. Post-procedural changes were mainly based on stent malapposition, under-expansion, or edge dissections, and resulted in additional stent deployment, post-dilatation, or both. Stent expansion, stent apposition, distal stent edge dissections, and reference lumen areas seem to be the most important stent optimization criteria for both decision-making as reducing the risk of adverse events during follow-up. In general, the incidence of procedural complications is low and not different from IVUS-guided or CAG-guided stenting. Also, clinical outcomes are comparable between OCT guidance and IVUS guidance. Both OCT and IVUS result in favorable clinical outcomes compared with CAG guidance alone. OCT-guided PCI improved strut coverage and stent apposition, and reduced tissue protrusions compared with CAG guidance only. OCT was non-inferior, but not superior, to IVUS guidance. There is no consensus about whether OCT guidance or IVUS guidance results in better stent expansion.
OCT results in a higher resolution than IVUS at the cost of a lower penetration depth. The penetration depth of OCT is an important disadvantage of OCT. Large lipid-rich plaques disable the ability to image the vessel border with OCT, due to signal attenuation. However, lumen dimensions can still be assessed. In addition, the presence of red thrombus results in signal-free shadowing, complicating image interpretation by OCT. The need for a contrast agent and the potential risk of CIN is another drawback of OCT. However, multiple OCT studies have shown low risks of CIN in patients treated with OCT. Thus, OCT can reveal more detail, where IVUS provides more insight in deeper layers of the coronary arteries. However, small abnormalities should be interpreted carefully. OCT and IVUS are complementary and should be considered depended on the case characteristics [34, 65].
The number of PCIs is rapidly increasing compared to surgical procedures [66]. Nowadays, PCIs are increasingly performed in more complex lesions and multivessel coronary disease. In addition, patients tend to be older with more calcifications. For example, many patients assigned to undergo transcatheter aortic valve implantation (TAVI) need prior revascularization. Due to the increasing complexity of the patient population and the limitations of CAG, ICI is becoming increasingly important. Hospitals with a large number of these complex patient cases potentially benefit the most from ICI. The patient population that should be assessed with ICI comprises patients with intermediate, complex, or ambiguous lesions. There is an unmistakable role for IVUS in left-main lesions and in patients with large lipid-rich plaques. OCT should especially be considered for lumen assessment and stent-related morphology. Especially OCT-guided stent optimization seems to result in better clinical outcomes. Table 2 and Fig. 1 show the situations and considerations in which ICI should be considered.
Summarizing figure for situations where intracoronary imaging should be considered (Table 2). (1) OCT or IVUS for pre-PCI stent sizing and identification of deployment site, OCT for lesion characterization: OCT image showing severe calcifications. (2) IVUS for ostial left-main lesion assessment and guidance: IVUS image showing left-main plaque. (3) Functional measurement (FFR/iFR) for uncertainty about severity of distal lesions and OCT for composition: iFR/FFR and OCT image showing intimal thickening. (4) OCT for bifurcation lesion assessment and guidance: OCT image showing a bifurcation with high resolution. (5) OCT for post-PCI stent assessment and optimization and assessment of stent failure: OCT images demonstrating malapposition (white arrow shows an intraluminal stent strut) and in-stent restenosis (yellow arrow shows a stent strut covered by neointima hyperplasia)
For some years, there has been discussion about the influence of gender on CAD. Men more frequently develop the disease and earlier in life. The incidence of CAD has been relatively low before the menopause, thereafter it increases rapidly. Munnur et al. (2016) reviewed the current literature on various subgroups and provided an overview of differences in clinical manifestations between genders [67]. Differences are especially seen in patients under the age of 65, whereafter plaque characteristics become more similar. In addition, women seem to benefit more from lipid-lowering therapy, in terms of plaque regression [68, 69]. Although differences in plaque characteristics exist between men and women, the occurrence of MACE seems similar [70]. As ICI provides more insight in plaque characteristics, it should be considered, regardless of gender. In addition, ICI is an essential tool when conducting research into CAD morphology in relation to gender and patient outcome.
Future Directions
In anticipation of the ILUMIEN IV trial and the OCTOBER trial, OCT is expected to improve clinical outcomes. However, it is still unknown which patients benefit the most from ICI. Further research should focus on which patients benefit the most from ICI, ideally differentiating between OCT and IVUS. Utilizing large datasets might support researchers. Luckily, large datasets of CAG and laboratory data already exist. New data is stored each day during treatment of patients with CAD. In addition, an increasing amount of ICI data is acquired. Such datasets might be used in the future for all kinds of research purposes.
Although speculative, ICI data might contribute to the development and training of prediction models, such as machine learning algorithms, to assess which patients benefit the most from ICI or to support physicians in deciding whether lesions should be stented or not. OCT could be more suitable for the development of such models, as OCT provides much higher resolutions than IVUS. Multiple studies already showed the potential of automatic interpretation of OCT images [71,72,73]. Other applications of OCT data might be in the further optimization of stent design. OCT can help to provide insight in the effects of different stent designs on in-stent restenosis or thrombosis. To conclude, the added value of OCT, in comparison with IVUS, probably lies especially in the optimization of PCI, both in a clinical as well as a research setting.
Conclusions
In conclusion, OCT is a safe procedure with few reported procedural complications. In general, OCT guidance seems to contribute to favorable clinical outcomes compared with CAG guidance only. However, in general, OCT guidance results in similar clinical outcomes as with IVUS guidance. Stent expansion, stent apposition, distal stent edge dissections, and reference lumen areas seem to be the most important stent optimization criteria for both decision-making and reducing the risk of adverse events during follow-up. OCT could be considered for lumen assessment and stent-related morphology in more complex cases in which CAG interpretation remains uncertain. Since OCT and IVUS have distinct characteristics, these techniques are complementary and should be considered carefully for each patient case based on the benefits and limitations of both techniques.
References
Lee CH, Hur SH. Optimization of percutaneous coronary intervention using optical coherence tomography. Korean Circ J. 2019;49(9):771–93.
Ali ZA, Maehara A, Genereux P, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet (London, England). 2016;388(10060):2618–28.
Gutierrez-Chico JL, Alegria-Barrero E, Teijeiro-Mestre R, et al. Optical coherence tomography: from research to practice. Eur Heart J Cardiovasc Imaging. 2012;13(5):370–84.
Mintz GS, Popma JJ, Pichard AD, et al. Limitations of angiography in the assessment of plaque distribution in coronary artery disease: a systematic study of target lesion eccentricity in 1446 lesions. Circulation. 1996;93(5):924–31.
Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165.
Yabushita H, Bouma BE, Houser SL, et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106(13):1640–5.
Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59(12):1058–72.
Ahn JM, Kang SJ, Yoon SH, et al. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drug-eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am J Cardiol. 2014;113(8):1338–477.
Elgendy IY, Mahmoud AN, Elgendy AY, Bavry AA. Outcomes with intravascular ultrasound-guided stent implantation: a meta-analysis of randomized trials in the era of drug-eluting stents. Circ Cardiovasc Interv. 2016;9(4):e003700.
Bavishi C, Sardar P, Chatterjee S, et al. Intravascular ultrasound-guided vs angiography-guided drug-eluting stent implantation in complex coronary lesions: meta-analysis of randomized trials. Am Heart J. 2017;185:26–34.
Nerlekar N, Cheshire CJ, Verma KP, et al. Intravascular ultrasound guidance improves clinical outcomes during implantation of both first- and second-generation drug-eluting stents: a meta-analysis. EuroIntervention. 2017;12(13):1632–42.
Zhang YJ, Pang S, Chen XY, et al. Comparison of intravascular ultrasound-guided versus angiography guided drug eluting stent implantation: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2015;15:153.
Koskinas KC, Nakamura M, Raber L, et al. Current use of intracoronary imaging in interventional practice-results of a European Association of Percutaneous Cardiovascular Interventions (EAPCI) and Japanese Association of Cardiovascular Interventions and Therapeutics (CVIT) clinical practice survey. Circ J. 2018;82(5):1360–8.
Popescu DP, Choo-Smith LP, Flueraru C, et al. Optical coherence tomography: fundamental principles, instrumental designs and biomedical applications. Biophys Rev. 2011;3(3):155.
Koganti S, Kotecha T, Rakhit RD. Choice of intracoronary imaging: when to use intravascular ultrasound or optical coherence tomography. Interv Cardiol (London, England). 2016;11(1):11–6.
Prati F, Regar E, Mintz GS, et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31(4):401–15.
Regar E, Ligthart J, Bruining N, van Soest G. The diagnostic value of intracoronary optical coherence tomography. Herz. 2011;36(5):417–29.
Maehara A, Ben-Yehuda O, Ali Z, et al. Comparison of stent expansion guided by optical coherence tomography versus intravascular ultrasound: the ILUMIEN II Study (Observational Study of optical coherence tomography [OCT] in patients undergoing fractional flow reserve [FFR] and percutaneous coronary intervention). JACC Cardiovasc Interv. 2015;8(13):1704–14.
Maehara A, Matsumura M, Ali ZA, Mintz GS, Stone GW. IVUS-guided versus OCT-guided coronary stent implantation: a critical appraisal. JACC Cardiovasc Imaging. 2017;10(12):1487–503.
Brezinski ME, Tearney GJ, Bouma BE, et al. Optical coherence tomography for optical biopsy. Properties and demonstration of vascular pathology. Circulation. 1996;93(6):1206–13.
Swanson EA, Huang D, Hee MR, Fujimoto JG, Lin CP, Puliafito CA. High-speed optical coherence domain reflectometry. Opt Lett. 1992;17(2):151–3.
Jang IK, Bouma BE, Kang DH, et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002;39(4):604–9.
Falk E. Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis. Characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Br Heart J. 1983;50(2):127–34.
Davies MJ. Detecting vulnerable coronary plaques. Lancet (London, England). 1996;347(9013):1422–3.
Lee RT, Libby P. The unstable atheroma. Arterioscler Thromb Vasc Biol. 1997;17(10):1859–67.
Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20(5):1262–75.
Naderi S. Spontaneous coronary artery dissection: an overview. Curr Atheroscler Rep. 2018;20(12):58.
Jackson R, Al-Hussaini A, Joseph S, et al. Spontaneous coronary artery dissection: pathophysiological insights from optical coherence tomography. JACC Cardiovas Imaging. 2019;12(12):2475–88.
Paulo M, Sandoval J, Lennie V, et al. Combined use of OCT and IVUS in spontaneous coronary artery dissection. JACC Cardiovasc Imaging. 2013;6(7):830–2.
Alfonso F, Canales E, Aleong G. Spontaneous coronary artery dissection: diagnosis by optical coherence tomography. Eur Heart J. 2009;30(3):385.
Alfonso F, Paulo M, Gonzalo N, et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol. 2012;59(12):1073–9.
Garcia-Guimaraes M, Bastante T, Antuna P, et al. Spontaneous coronary artery dissection: mechanisms, diagnosis and management. Eur Cardiol. 2020;15:1–8.
Kume T, Akasaka T, Kawamoto T, et al. Assessment of coronary arterial thrombus by optical coherence tomography. Am J Cardiol. 2006;97(12):1713–7.
Alfonso F, Dutary J, Paulo M, et al. Combined use of optical coherence tomography and intravascular ultrasound imaging in patients undergoing coronary interventions for stent thrombosis. Heart (Br Cardiac Soc). 2012;98(16):1213–20.
Jang IK, Tearney GJ, MacNeill B, et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. 2005;111(12):1551–5.
Wijns W, Shite J, Jones MR, et al. Optical coherence tomography imaging during percutaneous coronary intervention impacts physician decision-making: ILUMIEN I study. Eur Heart J. 2015;36(47):3346–55.
Leistner DM, Riedel M, Steinbeck L, et al. Real-time optical coherence tomography coregistration with angiography in percutaneous coronary intervention-impact on physician decision-making: the OPTICO-integration study. Catheteriz Cardiovasc Interv. 2018;92(1):30–7.
Meneveau N, Souteyrand G, Motreff P, et al. Optical coherence tomography to optimize results of percutaneous coronary intervention in patients with non-ST-elevation acute coronary syndrome: results of the multicenter, randomized DOCTORS study (does optical coherence tomography optimize results of stenting). Circulation. 2016;134(13):906–17.
Imola F, Mallus MT, Ramazzotti V, et al. Safety and feasibility of frequency domain optical coherence tomography to guide decision making in percutaneous coronary intervention. EuroIntervention. 2010;6(5):575–81.
Prati F, Di Vito L, Biondi-Zoccai G, et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l'Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention. 2012;8(7):823–9.
Ajj IJ, Zwaan EM, Oemrawsingh RM, et al. Appropriate use criteria for optical coherence tomography guidance in percutaneous coronary interventions: recommendations of the working group of interventional cardiology of the Netherlands Society of Cardiology. Netherlands Heart J. 2018;26(10):473–83.
Raber L, Mintz GS, Koskinas KC, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2018;39(35):3281–300.
de Jaegere P, Mudra H, Figulla H, et al. Intravascular ultrasound-guided optimized stent deployment. Immediate and 6 months clinical and angiographic results from the Multicenter Ultrasound Stenting in Coronaries Study (MUSIC Study). Eur Heart J. 1998;19(8):1214–23.
Habara M, Nasu K, Terashima M, et al. Impact of frequency-domain optical coherence tomography guidance for optimal coronary stent implantation in comparison with intravascular ultrasound guidance. Circ Cardiovasc Interv. 2012;5(2):193–201.
Antonsen L, Thayssen P, Maehara A, et al. Optical coherence tomography guided percutaneous coronary intervention with Nobori stent implantation in patients with non-ST-segment-elevation myocardial infarction (OCTACS) trial: difference in strut coverage and dynamic malapposition patterns at 6 months. Circ Cardiovasc Interv. 2015;8(8):e002446.
Kubo T, Shinke T, Okamura T, et al. Optical frequency domain imaging vs intravascular ultrasound in percutaneous coronary intervention (OPINION trial): study protocol for a randomized controlled trial. J Cardiol. 2016;68(5):455–60.
Kubo T, Shinke T, Okamura T, et al. Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): one-year angiographic and clinical results. Eur Heart J. 2017;38(42):3139–47.
Prati F, Guagliumi G, Mintz GS, et al. Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur Heart J. 2012;33(20):2513–20.
Prati F, Romagnoli E, Burzotta F, et al. Clinical impact of OCT findings during PCI: the CLI-OPCI II study. JACC Cardiovasc Imaging. 2015;8(11):1297–305.
Witzenbichler B, Maehara A, Weisz G, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. 2014;129(4):463–70.
Jang JS, Song YJ, Kang W, et al. Intravascular ultrasound-guided implantation of drug-eluting stents to improve outcome: a meta-analysis. JACC Cardiovasc Interv. 2014;7(3):233–43.
Hong SJ, Kim BK, Shin DH, et al. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: the IVUS-XPL randomized clinical trial. JAMA. 2015;314(20):2155–63.
Song HG, Kang SJ, Ahn JM, et al. Intravascular ultrasound assessment of optimal stent area to prevent in-stent restenosis after zotarolimus-, everolimus-, and sirolimus-eluting stent implantation. Catheteriz Cardiovasc Interv. 2014;83(6):873–8.
Stone GW, Witzenbichler B, Weisz G, et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet (London, England). 2013;382(9892):614–23.
Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. New Engl J Med. 2002;346(23):1773–800.
Finn AV, Joner M, Nakazawa G, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115(18):2435–41.
Otsuka F, Nakano M, Ladich E, Kolodgie FD, Virmani R. Pathologic etiologies of late and very late stent thrombosis following first-generation drug-eluting stent placement. Thrombosis. 2012;2012:608593.
Lee SY, Kim JS, Yoon HJ, et al. Early strut coverage in patients receiving drug-eluting stents and its implications for dual antiplatelet therapy: a randomized trial. JACC Cardiovasc imaging. 2018;11(12):1810–9.
Gatto L, Golino M, Marco V, et al. Role of optical coherence tomography in identifying sub-optimal stent positioning and predicting major adverse cardiac events in a comparative study with angiography: a CLIO-OPCI II sub-study. Coron Artery Dis. 2018;29(5):384–8.
Jones DA, Rathod KS, Koganti S, et al. Angiography alone versus angiography plus optical coherence tomography to guide percutaneous coronary intervention: outcomes from the pan-London PCI cohort. JACC Cardiovasc Interv. 2018;11(14):1313–21.
Iannaccone M, D'Ascenzo F, Frangieh AH, et al. Impact of an optical coherence tomography guided approach in acute coronary syndromes: a propensity matched analysis from the international FORMIDABLE-CARDIOGROUP IV and USZ registry. Catheteriz Cardiovasc Interv. 2017;90(2):E46–e52.
Ali Z, Landmesser U, Stone GW. ILUMIEN IV: OPTIMAL PCI 2019 [updated July 22, 2019. Available from: https://clinicaltrials.gov/ct2/show/NCT03507777].
Holm NR, Andreasen LN, Walsh S, et al. Rational and design of the European randomized optical coherence tomography optimized bifurcation event reduction trial (OCTOBER). Am Heart J. 2018;205:97–109.
Yamamoto MH, Maehara A, Song L, et al. Optical coherence tomography assessment of morphological characteristics in suspected coronary artery disease, but angiographically nonobstructive lesions. Cardiovasc Revasc Med. 2019;20(6):475–9.
Dash D. Optical coherence tomography is a kid on the block: I would choose intravascular ultrasound. Indian Heart J. 2017;69(3):407–10.
Leening MJ, Siregar S, Vaartjes I, et al. Heart disease in the Netherlands: a quantitative update. Netherlands Heart J. 2014;22(1):3–10.
Munnur RK, Nerlekar N, Wong DT. Imaging of coronary atherosclerosis in various susceptible groups. Cardiovasc Diagn Therapy. 2016;6(4):382–95.
Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet (London, England). 2010;376(9753):1670–81.
Puri R, Nissen SE, Shao M, et al. Sex-related differences of coronary atherosclerosis regression following maximally intensive statin therapy: insights from SATURN. JACC Cardiovasc Imaging. 2014;7(10):1013–22.
Lansky AJ, Ng VG, Maehara A, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5(3 Suppl):S62–72.
Abdolmanafi A, Duong L, Dahdah N, Adib IR, Cheriet F. Characterization of coronary artery pathological formations from OCT imaging using deep learning. Biomed Opt Express. 2018;9(10):4936–60.
Abdolmanafi A, Cheriet F, Duong L, Ibrahim R, Dahdah N. An automatic diagnostic system of coronary artery lesions in Kawasaki disease using intravascular optical coherence tomography imaging. J Biophoton. 2020;13(1):e201900112.
Lee J, Prabhu D, Kolluru C, et al. Fully automated plaque characterization in intravascular OCT images using hybrid convolutional and lumen morphology features. Sci Rep. 2020;10(1):2596.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. Figure 1 was designed by C.L.H. Broekmeulen in favor of the authors. OCT images were provided by Abbott Medical Nederland B.V.
Disclosures
The Department of Cardiology receives unrestricted grants from Biotronik (Berlin, Germany), Boston Scientific (Marlborough, Massachusetts) and Medtronic (Minneapolis, Minnesota). Timo T.M. Oosterveer, Sander M. van der Meer, Roderick W.C. Scherptong and J. Wouter Jukema have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Open Access
This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12425108.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Oosterveer, T.T.M., van der Meer, S.M., Scherptong, R.W.C. et al. Optical Coherence Tomography: Current Applications for the Assessment of Coronary Artery Disease and Guidance of Percutaneous Coronary Interventions. Cardiol Ther 9, 307–321 (2020). https://doi.org/10.1007/s40119-020-00185-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-020-00185-4