Abstract
FePt nanoparticles (NPs) and colloidal nanoparticle clusters (CNCs) have been synthesized by the reduction of platinum acetylacetonate (Pt(acac)2) combined with thermal decomposition of iron pentacarbonyl (Fe(CO)5) and compared to pure Pt NPs and CNCs for the catalytic activity of the oxygen reduction (ORR). The formation of NPs and CNCs has been carried out controlling injection temperature of the precursors and the surfactants during the synthesis. The size of the NPs and the CNCs formed is around 3 and 38 nm, respectively. High electrocatalytic performance of the FePt CNCs in comparison with that of nanoparticles and nanocluster of platinum has been obtained for the reduction reaction (ORR) in basic medium. The ORR is carried out by a four-electron charge transfer. The increase in the activity of the CNC structures formed by FePt nanoparticles can be attributed to the alloy formation that produces surface and electronic changes of the Pt atoms and to the structure of the CNCs obtained.

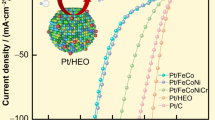
Polarization curves of (a) CNCs FePt, (b) NPs FePt, (c) CNCs Pt, and (d) NPs Pt and their morphology.







Similar content being viewed by others
References
Y.J. Wang, D.P. Wilkinson, J. Zhang, Chem. Rev. 7625, 111 (2011)
H. Zhang, P.K. Shen, Chem. Rev. 2780, 112 (2012)
C. Koenigsmann, E. Sutter, T.A. Chiesa, R.R. Adzic, S.S. Wong, Nano Lett. 2013, 12 (2012)
C. Koenigsmann, E. Sutter, R.R. Adzic, S.S. Wong, J. Phys. Chem. C 15297, 116 (2012)
A. Rabis, P. Rodriguez, T.J. Schmidt, ACS Catal. 864, 2 (2012)
J. Wu, L. Qi, H. You, A.M. Gross, J. Li, H. Yang, J. Am. Chem. Soc. 11880, 134 (2012)
G. Shaojun, L. Dongguo, Z. Huiyuan, Z. Sen, M. Nenad, M. Vojislav, R. Stamenkovic, S. Shouheng Angew, Chem. Int. Ed. 3465, 52 (2013)
H. Ataee-Esfahani, L. Wang, Y. Nemoto, Y. Yamauchi, Chem. Mater. 6310, 22 (2010)
W.Z. Li, Z.W. Chen, L.B. Xu, Y.S. Yan, J. Power Sources 2534, 195 (2010)
V. Mazumder, M.F. Chi, K.L. More, S.H. Sun, Angew. Chem. Int. Ed. 9368, 49 (2010)
F. Maroun, F. Ozanam, O.M. Magnussen, R.J. Behm, Science 1811, 293 (2001)
R. Burch, Acc. Chem. Res. 24, 15 (1982)
V.R. Stamenkovic, B.S. Mun, M. Arenz, K.J. Mayrhofer, C.A. Lukas, G.F. Wang, P.N. Ross, N.M. Markovic, Nat. Mater. 241, 6 (2007)
V.R. Stamenkovic, B. Fowler, B.S. Mun, G.F. Wang, P.N. Ross, C.A. Lucas, N.M. Markovic, Science 493, 315 (2007)
J. Wu, A. Gross, H. Yang, Nano Lett. 798, 11 (2011)
T. Toda, H. Igarashi, H. Uchida, M. Watanabe, J. Electrochem. Soc. 3750, 146 (1999)
G. Shaojun, L. Dongguo, Z. Huiyuan, Z. Sen, M.M. Nenad, R. Vojislav Stamenkovic, S. Shouheng, Angew. Chem. Int. Ed. 3465, 52 (2013)
K. Jaemin, L. Youngmin, S. Shouheng, J. Am. Chem. Soc. 4996, 132 (2010)
J. Snyder, I. McCue, K. Livi, J. Erlebacher, J. Am. Chem. Soc. 8633, 134 (2012)
L. Yizhong, C. Wei, J. Power Sources 107, 197 (2012)
W. Chen, S. Chen, Angew. Chem. Int. Ed. 2386, 48 (2009)
G. Fu, K. Wu, J. Lin, Y. Tang, Y. Chen, Y. Zhou, T. Lu, J. Phys. Chem. C 9826, 117 (2013)
S. Sun, C.B. Murray, D. Weller, L. Folks, A. Moser, Science 1989, 287 (2000)
Z. Lu, Y. Yin, Chem. Soc. Rev. 6874, 41 (2012)
X.W. Teng, H. Yang, J. Am. Chem. Soc. 14559, 125 (2003)
Z. Chen, M. Waje, W. Li, Y. Yan, Angew Chem 4138, 119 (2007)
D. Chen, X. Zhao, S. Chen, Y. Li, X. Fu, Q. Wu, S. Li, Y. Li, B.-L. Su, R.S. Ruoff, Carbon 755, 68 (2014)
J. Zeng, S. Liao, J. Yang Lee, Z. Liang, Inter J Hydrogen Energy 942, 35 (2010)
Acknowledgments
The work has been supported by the Spanish Ministry of Science and Innovation: MAT2012- 37109-C02 and 01.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Velasco, V., Ovejero, J.G., Crespo, P. et al. Comparison of FePt and Pt Nanostructures for Oxygen Reduction Reaction in Basic Medium. Electrocatalysis 7, 262–268 (2016). https://doi.org/10.1007/s12678-016-0305-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-016-0305-2