Abstract
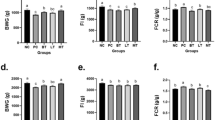
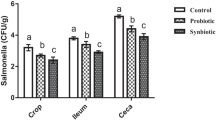
A strain of Bacillus subtilis (B. subtilis) BYS2 was previously isolated from Mount Tai, which is located in Tai’an City in the Shandong Province of China. The strain was then stored in the Environmental Microbiology Laboratory at Shandong Agricultural University. To evaluate the effect of the bacterium preparation in broiler production, we fed the bacterium (106 CFU/g) to 1-day-old broilers and continued this feeding for 6 weeks to analyze its effect on growth and immune performance. We found that the average weight of the bacterium-fed group increased by 17.19% at weeks 5 compared to the control group (P < 0.05). The height of the villi in the duodenum and jejunum and the ratio of villi to crypt were significantly increased in the bacterium-fed group at weeks 5 (P < 0.05). Also, the IgG in the serum of broilers in the experimental group increased by 31.60% (P < 0.05) and IgM 30.52% (P < 0.05) compared with those in the control group. The expressions of the major pattern recognition receptors (PRRs), antiviral proteins, pro-inflammatory cytokines, and β-defensins were significantly higher than those in the control group (P < 0.05). Meanwhile, the bursa immune organ indices of broilers in the experimental group were significantly higher than those in the control group (P < 0.05). Also, after 5 weeks of continuous feeding, when infected with Escherichia coli (E. coli) O1K1 and Newcastle disease virus (NDV) F48E8, the content of bacteria and virus in tissues and organs of the experimental group decreased significantly, and the survival rate of infected chickens increased by 31.1% and 17.7%, respectively (P < 0.05). These results show that the anti-infective B. subtilis BYS2 could, to some extent, replace antibiotics to promote growth, improve innate immunity, and enhance disease resistance in broilers.






Similar content being viewed by others
References
Wiseman A, Berman E, Klement E (2018) Risk factors for Newcastle disease in broiler farms in Israel. Prev Vet Med 149:92–97. https://doi.org/10.1016/j.prevetmed.2017.11.009
Hussain A, Shaik S, Ranjan A, Nandanwar N, Tiwari SK, Majid M, Baddam R, Qureshi IA, Semmler T, Wieler LH (2017) Risk of transmission of antimicrobial resistant Escherichia coli from commercial broiler and free-range retail chicken in India. Front Microbiol 8:2120. https://doi.org/10.3389/fmicb.2017.02120
Latorre JD, Hernandez-Velasco X, Kuttappan VA, Wolfenden RE, Vicente JL, Wolfenden AD, Bielke LR, Prado-Rebolledo OF, Morales E, Hargis BM (2015) Selection of Bacillus spp. for cellulase and xylanase production as direct-fed microbials to reduce digesta viscosity and Clostridium perfringens proliferation using an in vitro digestive model in different poultry diets. Front Vet Sci 2:25. https://doi.org/10.3389/fvets.2015.00025
Hu S, Wang L, Jiang Z (2017) Dietary additive probiotics modulation of the intestinal microbiota. Protein Pept Lett 24(5):382–387. https://doi.org/10.2174/0929866524666170223143615
Sadeghi AA, Shawrang P, Shakorzadeh S (2015) Immune response of Salmonella challenged broiler chickens fed diets containing Gallipro®, a Bacillus subtilis probiotic. Probiotics Antimicrob Proteins 7(1):24–30. https://doi.org/10.1007/s12602-014-9175-1
Ouwehand AC, Kirjavainen PV, Shortt C, Salminen S (1999) Probiotics: mechanisms and established effects. Int Dairy J 9(1):43–52. https://doi.org/10.1016/S0958-6946(99)00043-6
Rajput IR, Li WF, Li YL, Jian L, Wang MQ (2012) Application of probiotic (Bacillus subtilis) to enhance immunity, antioxidation, digestive enzymes activity and hematological profile of Shaoxing duck. Pak Vet J 33(1):69–72. https://doi.org/10.1111/vec.12017
Sarkar PK, Morrison E, Tinggi U, Somerset SM, Craven GS (1998) B-group vitamin and mineral contents of soybeans during kinema production. J Sci Food Agric 78(4):498–502. https://doi.org/10.1002/(sici)1097-0010(199812)78:4<498:aid-jsfa145>3.0.co;2-c
Sanders ME, Morelli L, Tompkins T (2003) Sporeformers as human probiotics: Bacillus, Sporolactobacillus, and Brevibacillus. Compr Rev Food Sci Food Saf 2(3):101–110. https://doi.org/10.1111/j.1541-4337.2003.tb00017.x
Additives EPo, Feed PoSuiA (2014) Scientific opinion on the safety and efficacy of vitamin B2 (80%) as riboflavin produced by Bacillus subtilis for all animal species, based on a dossier submitted by VITAC EEIG. EFSA J 12(1):3531. https://doi.org/10.2903/j.efsa.2014.3531
Gao Z, Wu H, Shi L, Zhang X, Sheng R, Yin F, Gooneratne R (2017) Study of Bacillus subtilis on growth performance, nutrition metabolism and intestinal microflora of 1 to 42 d broiler chickens. Anim Nutr 3(2):109–113. https://doi.org/10.1016/j.aninu.2017.02.002
Wang X, Yi Z, Ji C (2006) Effects of fructo-oligosaccharide and Bacillus subtilis on intestinal microflora, fecal emission of ammonia and sulfureted hydrogen and nutrient availability in broilers. Acta Vet Zootec Sin 37(4):337. https://doi.org/10.1360/aps040178
Xiumei D, Chaofan Z, Ping W (2004) The effect of compound probiotics on intestinal bacteria and anti-oxidation in broilers. China Poult 14. https://doi.org/10.16372/j.issn.1004-6364.2004.14.004
Stanley D, Hughes RJ, Moore RJ (2014) Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl Microbiol Biotechnol 98(10):4301–4310. https://doi.org/10.1007/s00253-014-5646-2
Guo J, Dong X, Liu S, Tong J (2018) High-throughput sequencing reveals the effect of Bacillus subtilis CGMCC 1.921 on the cecal microbiota and gene expression in ileum mucosa of laying hens. Poult Sci 97(7):2543–2556. https://doi.org/10.3382/ps/pey112
Al-Fataftah A-R, Abdelqader A (2014) Effects of dietary Bacillus subtilis on heat-stressed broilers performance, intestinal morphology and microflora composition. Anim Feed Sci Technol 198:279–285. https://doi.org/10.1016/j.anifeedsci.2014.10.012
Lee S, Ingale S, Kim J, Kim K, Lokhande A, Kim E, Kwon I, Kim Y, Chae B (2014) Effects of dietary supplementation with Bacillus subtilis LS 1–2 fermentation biomass on growth performance, nutrient digestibility, cecal microbiota and intestinal morphology of weanling pig. Anim Feed Sci Technol 188:102–110. https://doi.org/10.1016/j.anifeedsci.2013.12.001
Sen S, Ingale SL, Kim YW, Kim JS, Kim KH, Lohakare JD, Kim EK, Kim HS, Ryu MH, Kwon IK (2011) Effect of supplementation of Bacillus subtilis LS 1-2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res Vet Sci 93(1):264–268. https://doi.org/10.1016/j.rvsc.2011.05.021
Rajput IR, Li LY, Xin X, Wu BB, Juan ZL, Cui ZW, Yu DY, Li WF (2011) Effect of Saccharomyces boulardii and Bacillus subtilis B10 on intestinal ultrastructure modulation and mucosal immunity development mechanism in broiler chickens. Poult Sci 92(4):956–965. https://doi.org/10.3382/ps.2012-02845
Guo M, Wu F, Hao G, Qi Q, Li R, Li N, Wei L, Chai T (2017) Bacillus subtilis improves immunity and disease resistance in rabbits. Front Immunol 8:354. https://doi.org/10.3389/fimmu.2017.00354
Horgan R, Gavinelli A (2006) The expanding role of animal welfare within EU legislation and beyond. Livest Sci 103(3):0–307. https://doi.org/10.1016/j.livsci.2006.05.019
Pesti GM (1995) Nutrient requirements of poultry. Anim Feed Sci Technol 56(1):177–178. https://doi.org/10.1016/0377-8401(95)90024-1
Li R, Li N, Zhang J, Wang Y, Liu J, Cai Y, Chai T, Wei L (2016) Expression of immune-related genes of ducks infected with avian pathogenic Escherichia coli (APEC). Front Microbiol 7:637. https://doi.org/10.3389/fmicb.2016.00637
Reed LJ, Muench H (1938) A simple method of estimating fifty per cent endpoints. Am J Hyg 27(3):493-497. https://doi.org/10.1093/oxfordjournals.aje.a118408
Barri A, Honaker CF, Sottosanti JR, Hulet RM, Mcelroy AP (2011) Effect of incubation temperature on nutrient transporters and small intestine morphology of broiler chickens. Poult Sci 90(1):118-125. https://doi.org/10.3382/ps.2010-00908
Margo CE, Lee A (1995) Fixation of whole eyes: the role of fixative osmolarity in the production of tissue artifact. Graefes Arch Clin Exp Ophthalmol 233(6):366. https://doi.org/10.1007/BF00200486
Kim LM, King DJ, Suarez DL, Wong CW, Afonso CL (2007) Characterization of class I Newcastle disease virus isolates from Hong Kong live bird markets and detection using real-time reverse transcription-PCR. J Clin Microbiol 45(4):1310-1314. https://doi.org/10.1128/JCM.02594-06
Edens F (2003) An alternative for antibiotic se in poultry:probiotics. Braz J Poult Sci 5(2):75-97. https://doi.org/10.1590/S1516-635X2003000200001
Vogl C, Grill S, Schilling O, Stülke J, Mack M, Stolz J (2007) Characterization of riboflavin (vitamin B2) transport proteins from Bacillus subtilis and Corynebacterium glutamicum. J Bacteriol 189(20):7367-7375. https://doi.org/10.1128/JB.00590-07
Latorre JD, Hernandez-Velasco X, Kogut MH, Vicente JL, Wolfenden R, Wolfenden A, Hargis BM, Kuttappan VA, Tellez G (2014) Role of a Bacillus subtilis direct-fed microbial on digesta viscosity, bacterial translocation, and bone mineralization in turkey poults fed with a rye-based diet. Front Vet Sci 1:26. https://doi.org/10.3389/fvets.2014.00026
Hu Y, Dun Y, Li S, Zhao S, Peng N, Liang Y (2014) Effects of Bacillus subtilis KN-42 on growth performance, diarrhea and faecal bacterial flora of weaned piglets. Asian Austral J Anim Sci 27(8):1131-1140. https://doi.org/10.5713/ajas.2013.13737
Zhang Q, Ma H, Mai K, Zhang W, Liufu Z, Xu W (2010) Interaction of dietary Bacillus subtilis and fructooligosaccharide on the growth performance, non-specific immunity of sea cucumber, apostichopus japonicus. Fish Shellfish Immunol 29(2):204-211. https://doi.org/10.1016/j.fsi.2010.03.009
Uni Z, Perry GC (2006) Early development of small intestinal function. In: Graham CP (ed) Avian gut function in health and disease, 3rd. Hebrew, Israel, pp 29-42
Heckert R, Estevez I, Russek-Cohen E, Pettit-Riley R (2002) Effects of density and perch availability on the immune status of broilers. Poult Sci 81(4):451-457. https://doi.org/10.1093/ps/81.4.451
Koenen M, Kramer J, Van Der Hulst R, Heres L, Jeurissen S, Boersma W (2004) Immunomodulation by probiotic lactobacilli in layer-and meat-type chickens. Br Poult Sci 45(3):355-366. https://doi.org/10.1080/00071660410001730851
Samolińska W, Kowalczuk-Vasilev E, Grela ER (2018) Comparative effect of different dietary inulin sources and probiotics on growth performance and blood characteristics in growing-finishing pigs. Arch Anim Nutr 72(5):379-395. https://doi.org/10.1080/1745039X.2018.1505147
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124(4):783-801. https://doi.org/10.1016/j.cell.2006.02.015
Medzhitov R (2001) Toll-like receptors and innate immunity. Nat Rev Immunol 1(2):135-145. https://doi.org/10.4167/jbv.2011.41.4.225
Wilden H, Schirrmacher V, Fournier P (2011) Important role of interferon regulatory factor (IRF)-3 in the interferon response of mouse macrophages upon infection by Newcastle disease virus. Int J Oncol 39(2):493-504. https://doi.org/10.3892/ijo.2011.1033
Andrejeva J, Childs K, Young D, Carlos T, Stock N, Goodbourn S, Randall R (2004) The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-β promoter. Proc Natl Acad Sci U S A 101(49):17264-17269. https://doi.org/10.1073/pnas.0407639101
D-c K, Gopalkrishnan RV, Wu Q, Jankowsky E, Pyle AM, Fisher PB (2002) mda-5: an interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc Natl Acad Sci U S A 99(2):637-642. https://doi.org/10.1073/pnas.022637199
Wei L, Cui J, Song Y, Zhang S, Han F, Yuan R, Gong L, Jiao P, Liao M (2014) Duck MDA5 functions in innate immunity against H5N1 highly pathogenic avian influenza virus infections. Vet Res 45(1):66. https://doi.org/10.1186/1297-9716-45-66
Wang Z, Zhang P, Fu W, Zhang Y, Li T, Pan B, Wei P (2010) Effect of probiotics on Newcastle disease virus. Acta Microbiol Sin 50(12):1664-1669. https://doi.org/10.13343/j.cnki.wsxb.2010.12.013
Shahir MH, Afsarian O, Ghasemi S, Tellez G (2014) Effects of dietary inclusion of probiotic or prebiotic on growth performance, organ weight, blood parameters and antibody titers against influenza and Newcastle in broiler chickens. Int J Poult Sci 13(2):70. https://doi.org/10.3923/ijps.2014.70.75
Wehkamp J, Harder J, Wehkamp K, Wehkamp-von Meissner B, Schlee M, Enders C, Sonnenborn U, Nuding S, Bengmark S, Fellermann K (2004) NF-κB-and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun 72(10):5750-5758. https://doi.org/10.1128/IAI.72.10.5750-5758.2004
Mageed AA, Isobe N, Yoshimura Y (2008) Expression of avian β-defensins in the oviduct and effects of lipopolysaccharide on their expression in the vagina of hens. Poult Sci 87(5):979-984. https://doi.org/10.1637/10848-042014-Reg.1
Quinteiro-Filho WM, Calefi AS, Cruz DSG, Aloia TP, Zager A, Astolfi-Ferreira CS, Ferreira JAP, Sharif S, Palermo-Neto J (2017) Heat stress decreases expression of the cytokines, avian β-defensins 4 and 6 and toll like receptor 2 in broiler chickens infected with Salmonella Enteritidis. Vet Immunol Immunopathol 186:19-28. https://doi.org/10.1016/j.vetimm.2017.02.006
Sandford EE (2011) Spleen transcriptome response to infection with avian pathogenic Escherichia coli (APEC) in broiler chickens. BMC Genomics 12(1):469. https://doi.org/10.1186/1471-2164-12-469
Wang Y-G, Fang W-L, Wei J, Wang T, Wang N, Ma J-L, Shi M (2013) The involvement of NLRX1 and NLRP3 in the development of nonalcoholic steatohepatitis in mice. J Chin Med Assoc 76(12):686-692. https://doi.org/10.1016/j.jcma.2013.08.010
Lee MO, Jang H-J, Rengaraj D, Yang S-Y, Han JY, Lamont SJ, Womack JE (2016) Tissue expression and antibacterial activity of host defense peptides in chicken. BMC Vet Res 12(1):231. https://doi.org/10.1186/s12917-016-0866-6
Zhang Q, Tan B, Mai K, Zhang W, Ma H, Ai Q, Wang X, Liufu Z (2011) Dietary administration of Bacillus (B. licheniformis and B. subtilis) and isomaltooligosaccharide influences the intestinal microflora, immunological parameters and resistance against Vibrio alginolyticus in shrimp, Penaeus japonicus (Decapoda: Penaeidae). Aquac Res 42(7):943-952. https://doi.org/10.1111/j.1365-2109.2010.02677.x
Li Z, Ming Y, Michael Z, Shuping Z (2014) Expression, purification, and in vitro comparative characterization of avian beta-defensin-2, -6, and -12. Avian Dis 58(4):541-549. https://doi.org/10.1637/10848-042014-Reg.1
Yang M, Zhang C, Zhang X, Zhang MZ, Rottinghaus GE, Zhang S (2016) Structure-function analysis of avian β-defensin-6 and β-defensin-12: role of charge and disulfide bridges. BMC Microbiol 16(1):210. https://doi.org/10.1186/s12866-016-0828-y
Tellez G, Latorre JD (2017) Alternatives to antimicrobial growth promoters and their impact in gut microbiota, health and disease. Front Vet Sci 4:196. https://doi.org/10.3389/fvets.2017.00196
Kan S, Nanno M (2008) Probiotics and immunology: separating the wheat from the chaff. Trends Immunol 29(11):565-573. https://doi.org/10.1016/j.it.2008.07.011
Latorre JD, Hernandez-Velasco X, Wolfenden RE, Vicente JL, Wolfenden AD, Menconi A, Bielke LR, Hargis BM, Tellez G (2016) Evaluation and selection of Bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as direct-fed microbial candidates for poultry. Front Vet Sci 3:95. https://doi.org/10.3389/fvets.2016.00095
Funding
This study was sponsored by the key research project of Shandong Province “Research of a new probiotics for anti-infection, environmental improvement and alternative antibiotic and their products development” (2016GNC110014).
Author information
Authors and Affiliations
Contributions
YD and RL designed and conducted the study, performed most of the experiments, and wrote the manuscript. LM and YL performed the calculation. JZ and XQ collected samples. BW and TC discussed the results and revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Statement
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dong, Y., Li, R., Liu, Y. et al. Benefit of Dietary Supplementation with Bacillus subtilis BYS2 on Growth Performance, Immune Response, and Disease Resistance of Broilers. Probiotics & Antimicro. Prot. 12, 1385–1397 (2020). https://doi.org/10.1007/s12602-020-09643-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-020-09643-w