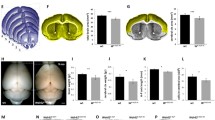
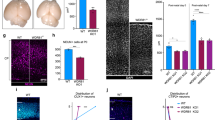
Abstract
Genetic disruptions of spindle/centrosome-associated WD40-repeat protein 62 (WDR62) are causative for autosomal recessive primary microcephaly (MCPH) and a broader range of cortical malformations. Since the identification of WDR62 as encoded by the MCPH2 locus in 2010, recent studies that have deleted/depleted WDR62 in various animal models of cortical development have highlighted conserved functions in brain growth. Here, we provide a timely review of our current understanding of WDR62 contributions in the self-renewal, expansion and fate specification of neural stem and progenitor cells that are critical for neocortical development. Recent studies have revealed multiple functions for WDR62 in the regulation of spindle organization, mitotic progression and the duplication and biased inheritance of centrosomes during asymmetric divisions. We also discuss recently elaborated WDR62 interaction partners that include Aurora and c-Jun N-terminal kinases as part of complex signalling mechanisms that may define its neural functions. These studies provide new insights into the molecular and cellular processes that are required for brain formation and implicated in the genesis of primary microcephaly.





Similar content being viewed by others
References
Bilguvar K, Ozturk AK, Louvi A, Kwan KY, Choi M, Tatli B, Yalnizoglu D, Tuysuz B et al (2010) Whole-exome sequencing identifies recessive WDR62 mutations in severe brain malformations. Nature 467(7312):207–210. https://doi.org/10.1038/nature09327
Nicholas AK, Khurshid M, Desir J, Carvalho OP, Cox JJ, Thornton G, Kausar R, Ansar M et al (2010) WDR62 is associated with the spindle pole and is mutated in human microcephaly. Nat Genet 42(11):1010–1014. https://doi.org/10.1038/ng.682
Yu TW, Mochida GH, Tischfield DJ, Sgaier SK, Flores-Sarnat L, Sergi CM, Topcu M, McDonald MT et al (2010) Mutations in WDR62, encoding a centrosome-associated protein, cause microcephaly with simplified gyri and abnormal cortical architecture. Nat Genet 42(11):1015–1020. https://doi.org/10.1038/ng.683
Bogoyevitch MA, Yeap YY, Qu Z, Ngoei KR, Yip YY, Zhao TT, Heng JI, Ng DC (2012) WD40-repeat protein 62 is a JNK-phosphorylated spindle pole protein required for spindle maintenance and timely mitotic progression. J Cell Sci 125(Pt 21):5096–5109. https://doi.org/10.1242/jcs.107326
Chen JF, Zhang Y, Wilde J, Hansen KC, Lai F, Niswander L (2014) Microcephaly disease gene Wdr62 regulates mitotic progression of embryonic neural stem cells and brain size. Nat Commun 5:3885. https://doi.org/10.1038/ncomms4885
Jayaraman D, Kodani A, Gonzalez DM, Mancias JD, Mochida GH, Vagnoni C, Johnson J, Krogan N et al (2016) Microcephaly proteins Wdr62 and Aspm define a mother centriole complex regulating centriole biogenesis, apical complex, and cell fate. Neuron 92(4):813–828. https://doi.org/10.1016/j.neuron.2016.09.056
Gilmore EC, Walsh CA (2013) Genetic causes of microcephaly and lessons for neuronal development. WIREs Dev Biol 2(4):461–478. https://doi.org/10.1002/wdev.89
Morris-Rosendahl DJ, Kaindl AM (2015) What next-generation sequencing (NGS) technology has enabled us to learn about primary autosomal recessive microcephaly (MCPH). Mol Cell Probes 29(5):271–281. https://doi.org/10.1016/j.mcp.2015.05.015
Wasserman T, Katsenelson K, Daniliuc S, Hasin T, Choder M, Aronheim A (2010) A novel c-Jun N-terminal kinase (JNK)-binding protein WDR62 is recruited to stress granules and mediates a nonclassical JNK activation. Mol Biol Cell 21(1):117–130. https://doi.org/10.1091/mbc.E09-06-0512
Woods CG, Parker A (2013) Investigating microcephaly. Arch Dis Child 98(9):707–713. https://doi.org/10.1136/archdischild-2012-302882
Woods CG, Bond J, Enard W (2005) Autosomal recessive primary microcephaly (MCPH): a review of clinical, molecular, and evolutionary findings. Am J Hum Genet 76(5):717–728. https://doi.org/10.1086/429930
Zaqout S, Morris-Rosendahl D, Kaindl AM (2017) Autosomal recessive primary microcephaly (MCPH): an update. Neuropediatrics 48(3):135–142. https://doi.org/10.1055/s-0037-1601448
Roberts E, Jackson AP, Carradice AC, Deeble VJ, Mannan J, Rashid Y, Jafri H, McHale DP et al (1999) The second locus for autosomal recessive primary microcephaly (MCPH2) maps to chromosome 19q13.1-13.2. Eur J Hum Genet 7(7):815–820. https://doi.org/10.1038/sj.ejhg.5200385
Pervaiz N, Abbasi AA (2016) Molecular evolution of WDR62, a gene that regulates neocorticogenesis. Meta Gene 9:1–9. https://doi.org/10.1016/j.mgene.2016.02.005
Ramdas Nair A, Singh P, Salvador Garcia D, Rodriguez-Crespo D, Egger B, Cabernard C (2016) The microcephaly-associated protein Wdr62/CG7337 is required to maintain centrosome asymmetry in drosophila neuroblasts. Cell Rep 14(5):1100–1113. https://doi.org/10.1016/j.celrep.2015.12.097
Banerjee S, Chen H, Huang H, Wu J, Yang Z, Deng W, Chen D, Deng J et al (2016) Novel mutations c.28G>T (p.Ala10Ser) and c.189G>T (p.Glu63Asp) in WDR62 associated with early onset acanthosis and hyperkeratosis in a patient with autosomal recessive microcephaly type 2. Oncotarget 7(48):78363–78371. 10.18632/oncotarget.13279
Ahmad I, Baig SM, Abdulkareem AR, Hussain MS, Sur I, Toliat MR, Nurnberg G, Dalibor N et al (2016) Genetic heterogeneity in Pakistani microcephaly families revisited. Clin Genet 92(1):62–68. https://doi.org/10.1111/cge.12955
Sajid Hussain M, Marriam Bakhtiar S, Farooq M, Anjum I, Janzen E, Reza Toliat M, Eiberg H, Kjaer KW et al (2013) Genetic heterogeneity in Pakistani microcephaly families. Clin Genet 83(5):446–451. https://doi.org/10.1111/j.1399-0004.2012.01932.x
Bastaki F, Mohamed M, Nair P, Saif F, Tawfiq N, Aithala G, El-Halik M, Al-Ali M et al (2016) Novel splice-site mutation in WDR62 revealed by whole-exome sequencing in a Sudanese family with primary microcephaly. Congenit Anom 56(3):135–137. https://doi.org/10.1111/cga.12144
Bhat V, Girimaji SC, Mohan G, Arvinda HR, Singhmar P, Duvvari MR, Kumar A (2011) Mutations in WDR62, encoding a centrosomal and nuclear protein, in Indian primary microcephaly families with cortical malformations. Clin Genet 80(6):532–540. https://doi.org/10.1111/j.1399-0004.2011.01686.x
Memon MM, Raza SI, Basit S, Kousar R, Ahmad W, Ansar M (2013) A novel WDR62 mutation causes primary microcephaly in a Pakistani family. Mol Biol Rep 40(1):591–595. https://doi.org/10.1007/s11033-012-2097-7
Kousar R, Hassan MJ, Khan B, Basit S, Mahmood S, Mir A, Ahmad W, Ansar M (2011) Mutations in WDR62 gene in Pakistani families with autosomal recessive primary microcephaly. BMC Neurol 11:119. https://doi.org/10.1186/1471-2377-11-119
Farag HG, Froehler S, Oexle K, Ravindran E, Schindler D, Staab T, Huebner A, Kraemer N et al (2013) Abnormal centrosome and spindle morphology in a patient with autosomal recessive primary microcephaly type 2 due to compound heterozygous WDR62 gene mutation. Orphanet J Rare Dis 8:178. https://doi.org/10.1186/1750-1172-8-178
Poulton CJ, Schot R, Seufert K, Lequin MH, Accogli A, Annunzio GD, Villard L, Philip N et al (2014) Severe presentation of WDR62 mutation: is there a role for modifying genetic factors? Am J Med Genet A 164A(9):2161–2171. https://doi.org/10.1002/ajmg.a.36611
Murdock DR, Clark GD, Bainbridge MN, Newsham I, Wu YQ, Muzny DM, Cheung SW, Gibbs RA et al (2011) Whole-exome sequencing identifies compound heterozygous mutations in WDR62 in siblings with recurrent polymicrogyria. Am J Med Genet A 155A(9):2071–2077. https://doi.org/10.1002/ajmg.a.34165
Najmabadi H, Hu H, Garshasbi M, Zemojtel T, Abedini SS, Chen W, Hosseini M, Behjati F et al (2011) Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature 478(7367):57–63. https://doi.org/10.1038/nature10423
Rupp V, Rauf S, Naveed I, Windpassinger C, Mir A (2014) A novel single base pair duplication in WDR62 causes primary microcephaly. BMC Med Genet 15:107. https://doi.org/10.1186/s12881-014-0107-4
Naseer MI, Rasool M, Sogaty S, Chaudhary RA, Mansour HM, Chaudhary AG, Abuzenadah AM, Al-Qahtani MH (2017) A novel WDR62 mutation causes primary microcephaly in a large consanguineous Saudi family. Ann Saudi Med 37(2):148–153. https://doi.org/10.5144/0256-4947.2017.148
Xu D, Zhang F, Wang Y, Sun Y, Xu Z (2014) Microcephaly-associated protein WDR62 regulates neurogenesis through JNK1 in the developing neocortex. Cell Rep 6(1):104–116. https://doi.org/10.1016/j.celrep.2013.12.016
Cohen-Katsenelson K, Wasserman T, Darlyuk-Saadon I, Rabner A, Glaser F, Aronheim A (2013) Identification and analysis of a novel dimerization domain shared by various members of c-Jun N-terminal kinase (JNK) scaffold proteins. J Biol Chem 288(10):7294–7304. https://doi.org/10.1074/jbc.M112.422055
Cohen-Katsenelson K, Wasserman T, Khateb S, Whitmarsh AJ, Aronheim A (2011) Docking interactions of the JNK scaffold protein WDR62. Biochem J 439(3):381–390. https://doi.org/10.1042/BJ20110284
Novorol C, Burkhardt J, Wood KJ, Iqbal A, Roque C, Coutts N, Almeida AD, He J et al (2013) Microcephaly models in the developing zebrafish retinal neuroepithelium point to an underlying defect in metaphase progression. Open Biol 3(10):130065. https://doi.org/10.1098/rsob.130065
Sgourdou P, Mishra-Gorur K, Saotome I, Henagariu O, Tuysuz B, Campos C, Ishigame K, Giannikou K et al (2017) Disruptions in asymmetric centrosome inheritance and WDR62-Aurora kinase B interactions in primary microcephaly. Sci Rep 7:43708. https://doi.org/10.1038/srep43708
Lim NR, Shohayeb B, Zaytseva O, Mitchell N, Millard SS, Ng DC, Quinn LM (2017) Glial-specific functions of microcephaly protein WDR62, and interaction with the mitotic kinase AURKA, are essential for Drosophila brain growth. Stem Cell Rep S2213-6711(17):30222–30229. https://doi.org/10.1016/j.stemcr.2017.05.015
Florio M, Huttner WB (2014) Neural progenitors, neurogenesis and the evolution of the neocortex. Development 141(11):2182–2194. https://doi.org/10.1242/dev.090571
Pollen AA, Nowakowski TJ, Chen J, Retallack H, Sandoval-Espinosa C, Nicholas CR, Shuga J, Liu SJ et al (2015) Molecular identity of human outer radial glia during cortical development. Cell 163(1):55–67. https://doi.org/10.1016/j.cell.2015.09.004
Cunningham CL, Martinez-Cerdeno V, Noctor SC (2013) Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci 33(10):4216–4233. https://doi.org/10.1523/JNEUROSCI.3441-12.2013
Fietz SA, Huttner WB (2011) Cortical progenitor expansion, self-renewal and neurogenesis-a polarized perspective. Curr Opin Neurobiol 21(1):23–35. https://doi.org/10.1016/j.conb.2010.10.002
Lesage B, Gutierrez I, Marti E, Gonzalez C (2010) Neural stem cells: the need for a proper orientation. Curr Opin Genet Dev 20(4):438–442. https://doi.org/10.1016/j.gde.2010.04.013
Manzini MC, Walsh CA (2011) What disorders of cortical development tell us about the cortex: one plus one does not always make two. Curr Opin Genet Dev 21(3):333–339. https://doi.org/10.1016/j.gde.2011.01.006
Alshawaf A, Antonic A, Skafidas E, Ng DCH, Dottori M (2017) WDR62 regulates early neural and glial progenitor specification from human pluripotent stem cells. Stem Cells Int 2017:7848932. https://doi.org/10.1155/2017/7848932
Koyano S, Ito M, Takamatsu N, Shiba T, Yamamoto K, Yoshioka K (1999) A novel Jun N-terminal kinase (JNK)-binding protein that enhances the activation of JNK by MEK kinase 1 and TGF-beta-activated kinase 1. FEBS Lett 457(3):385–388
Stirnimann CU, Petsalaki E, Russell RB, Muller CW (2010) WD40 proteins propel cellular networks. Trends Biochem Sci 35(10):565–574. https://doi.org/10.1016/j.tibs.2010.04.003
Macia MS, Halbritter J, Delous M, Bredrup C, Gutter A, Filhol E, Mellgren AE, Leh S et al (2017) Mutations in MAPKBP1 cause juvenile or late-onset cilia-independent nephronophthisis. Am J Human Genet 100(2):323–333. https://doi.org/10.1016/j.ajhg.2016.12.011
Hadad M, Aviram S, Darlyuk-Saadon I, Cohen-Katsenelson K, Whitmarsh AJ, Aronheim A (2015) The association of the JNK scaffold protein, WDR62, with the mixed lineage kinase 3, MLK3. MAP Kinase 4(1):5307
Zhang F, Yu J, Yang T, Xu D, Chi Z, Xia Y, Xu Z (2016) A novel c-Jun N-terminal kinase (JNK) signaling complex involved in neuronal migration during brain development. J Biol Chem 291(22):11466–11475. https://doi.org/10.1074/jbc.M116.716811
Kodani A, Yu TW, Johnson JR, Jayaraman D, Johnson TL, Al-Gazali L, Sztriha L, Partlow JN (2015) Centriolar satellites assemble centrosomal microcephaly proteins to recruit CDK2 and promote centriole duplication. eLife 4, e07519. https://doi.org/10.7554/eLife.07519
Lim NR, Yeap YY, Ang CS, Williamson NA, Bogoyevitch MA, Quinn LM, Ng DC (2016) Aurora A phosphorylation of WD40-repeat protein 62 in mitotic spindle regulation. Cell Cycle 15(3):413–424. https://doi.org/10.1080/15384101.2015.1127472
Lim NR, Yeap YY, Zhao TT, Yip YY, Wong SC, Xu D, Ang CS, Williamson NA et al (2015) Opposing roles for JNK and Aurora A in regulating the association of WDR62 with spindle microtubules. J Cell Sci 128(3):527–540. https://doi.org/10.1242/jcs.157537
Bogoyevitch MA, Ngoei KR, Zhao TT, Yeap YY, Ng DC (2010) c-Jun N-terminal kinase (JNK) signaling: recent advances and challenges. Biochim Biophys Acta 1804(3):463–475. https://doi.org/10.1016/j.bbapap.2009.11.002
Guarguaglini G, Duncan PI, Stierhof YD, Holmstrom T, Duensing S, Nigg EA (2005) The forkhead-associated domain protein Cep170 interacts with Polo-like kinase 1 and serves as a marker for mature centrioles. Mol Biol Cell 16(3):1095–1107. https://doi.org/10.1091/mbc.E04-10-0939
Rome P, Montembault E, Franck N, Pascal A, Glover DM, Giet R (2010) Aurora A contributes to p150(glued) phosphorylation and function during mitosis. J Cell Biol 189(4):651–659. https://doi.org/10.1083/jcb.201001144
Kettenbach AN, Schweppe DK, Faherty BK, Pechenick D, Pletnev AA, Gerber SA (2011) Quantitative phosphoproteomics identifies substrates and functional modules of Aurora and Polo-like kinase activities in mitotic cells. Sci Signal 4(179):rs5. https://doi.org/10.1126/scisignal.2001497
Barr AR, Gergely F (2007) Aurora-A: the maker and breaker of spindle poles. J Cell Sci 120(Pt 17):2987–2996. https://doi.org/10.1242/jcs.013136
Lee CY, Andersen RO, Cabernard C, Manning L, Tran KD, Lanskey MJ, Bashirullah A, Doe CQ (2006) Drosophila Aurora-A kinase inhibits neuroblast self-renewal by regulating aPKC/Numb cortical polarity and spindle orientation. Genes Dev 20(24):3464–3474. https://doi.org/10.1101/gad.1489406
Wang H, Somers GW, Bashirullah A, Heberlein U, Yu F, Chia W (2006) Aurora-A acts as a tumor suppressor and regulates self-renewal of Drosophila neuroblasts. Genes Dev 20(24):3453–3463. https://doi.org/10.1101/gad.1487506
Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Barres BA, Rowitch DH (2012) Astrocytes and disease: a neurodevelopmental perspective. Genes Dev 26(9):891–907. https://doi.org/10.1101/gad.188326.112
Sfakianos AP, Whitmarsh AJ, Ashe MP (2016) Ribonucleoprotein bodies are phased in. Biochem Soc Trans 44(5):1411–1416. https://doi.org/10.1042/BST20160117
Antoniou X, Borsello T (2012) The JNK signalling transduction pathway in the brain. Front Biosci 4:2110–2120
Sabapathy K (2012) Role of the JNK pathway in human diseases. Prog Mol Biol Transl Sci 106:145–169. https://doi.org/10.1016/B978-0-12-396456-4.00013-4
Sabapathy K, Jochum W, Hochedlinger K, Chang L, Karin M, Wagner EF (1999) Defective neural tube morphogenesis and altered apoptosis in the absence of both JNK1 and JNK2. Mech Dev 89(1–2):115–124
Westerlund N, Zdrojewska J, Padzik A, Komulainen E, Bjorkblom B, Rannikko E, Tararuk T, Garcia-Frigola C et al (2011) Phosphorylation of SCG10/stathmin-2 determines multipolar stage exit and neuronal migration rate. Nat Neurosci 14(3):305–313. https://doi.org/10.1038/nn.2755
Barr AR, Kilmartin JV, Gergely F (2010) CDK5RAP2 functions in centrosome to spindle pole attachment and DNA damage response. J Cell Biol 189(1):23–39. https://doi.org/10.1083/jcb.200912163
Knoblich JA (2008) Mechanisms of asymmetric stem cell division. Cell 132(4):583–597. https://doi.org/10.1016/j.cell.2008.02.007
Siller KH, Doe CQ (2008) Lis1/dynactin regulates metaphase spindle orientation in Drosophila neuroblasts. Dev Biol 319(1):1–9. https://doi.org/10.1016/j.ydbio.2008.03.018
Januschke J, Nathke I (2014) Stem cell decisions: a twist of fate or a niche market? Semin Cell Dev Biol 34:116–123. https://doi.org/10.1016/j.semcdb.2014.02.014
Insolera R, Bazzi H, Shao W, Anderson KV, Shi SH (2014) Cortical neurogenesis in the absence of centrioles. Nat Neurosci 17(11):1528–1535. https://doi.org/10.1038/nn.3831
Marjanovic M, Sanchez-Huertas C, Terre B, Gomez R, Scheel JF, Pacheco S, Knobel PA, Martinez-Marchal A et al (2015) CEP63 deficiency promotes p53-dependent microcephaly and reveals a role for the centrosome in meiotic recombination. Nat Commun 6:7676. https://doi.org/10.1038/ncomms8676
Kim S, Lehtinen MK, Sessa A, Zappaterra MW, Cho SH, Gonzalez D, Boggan B, Austin CA et al (2010) The apical complex couples cell fate and cell survival to cerebral cortical development. Neuron 66(1):69–84. https://doi.org/10.1016/j.neuron.2010.03.019
Higginbotham H, Guo J, Yokota Y, Umberger NL, Su CY, Li J, Verma N, Hirt J et al (2013) Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation. Nat Neurosci 16(8):1000–1007. https://doi.org/10.1038/nn.3451
Tong CK, Han YG, Shah JK, Obernier K, Guinto CD, Alvarez-Buylla A (2014) Primary cilia are required in a unique subpopulation of neural progenitors. Proc Natl Acad Sci U S A 111(34):12438–12443. https://doi.org/10.1073/pnas.1321425111
Paridaen JT, Wilsch-Brauninger M, Huttner WB (2013) Asymmetric inheritance of centrosome-associated primary cilium membrane directs ciliogenesis after cell division. Cell 155(2):333–344. https://doi.org/10.1016/j.cell.2013.08.060
Pelletier L, Yamashita YM (2012) Centrosome asymmetry and inheritance during animal development. Curr Opin Cell Biol 24(4):541–546. https://doi.org/10.1016/j.ceb.2012.05.005
Conduit PT, Raff JW (2010) Cnn dynamics drive centrosome size asymmetry to ensure daughter centriole retention in Drosophila neuroblasts. Curr Biol 20(24):2187–2192. https://doi.org/10.1016/j.cub.2010.11.055
Wang X, Tsai JW, Imai JH, Lian WN, Vallee RB, Shi SH (2009) Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature 461(7266):947–955. https://doi.org/10.1038/nature08435
Zhang Y, Tian Y, Yu JJ, He J, Luo J, Zhang S, Tang CE, Tao YM (2013) Overexpression of WDR62 is associated with centrosome amplification in human ovarian cancer. J Ovar Res 6(1):55. https://doi.org/10.1186/1757-2215-6-55
Liang Y, Gao H, Lin SY, Peng G, Huang X, Zhang P, Goss JA, Brunicardi FC et al (2010) BRIT1/MCPH1 is essential for mitotic and meiotic recombination DNA repair and maintaining genomic stability in mice. PLoS Genet 6(1):e1000826. https://doi.org/10.1371/journal.pgen.1000826
Pulvers JN, Bryk J, Fish JL, Wilsch-Brauninger M, Arai Y, Schreier D, Naumann R, Helppi J et al (2010) Mutations in mouse Aspm (abnormal spindle-like microcephaly associated) cause not only microcephaly but also major defects in the germline. Proc Natl Acad Sci U S A 107(38):16595–16600. https://doi.org/10.1073/pnas.1010494107
Zaqout S, Bessa P, Kramer N, Stoltenburg-Didinger G, Kaindl AM (2017) CDK5RAP2 is required to maintain the germ cell pool during embryonic development. Stem Cell Rep 8(2):198–204. https://doi.org/10.1016/j.stemcr.2017.01.002
Shinmura K, Kato H, Kawanishi Y, Igarashi H, Inoue Y, Yoshimura K, Nakamura S, Fujita H et al (2017) WDR62 overexpression is associated with a poor prognosis in patients with lung adenocarcinoma. Mol Carcinog 56(8):1984–1991. https://doi.org/10.1002/mc.22647
Zeng S, Tao Y, Huang J, Zhang S, Shen L, Yang H, Pei H, Zhong M et al (2013) WD40 repeat-containing 62 overexpression as a novel indicator of poor prognosis for human gastric cancer. Eur J Cancer 49(17):3752–3762. https://doi.org/10.1016/j.ejca.2013.07.015
Acknowledgements
DN acknowledges funding support from the National Health and Medical Research Council (APP1046032), Australian Research Council (FT120100193) and Cancer Council (APP1101931). NL was a recipient of a Melbourne International Research Scholarship from the University of Melbourne and BS is a recipient of a UQ International Scholarship from the University of Queensland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shohayeb, B., Lim, N.R., Ho, U. et al. The Role of WD40-Repeat Protein 62 (MCPH2) in Brain Growth: Diverse Molecular and Cellular Mechanisms Required for Cortical Development. Mol Neurobiol 55, 5409–5424 (2018). https://doi.org/10.1007/s12035-017-0778-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0778-x