Abstract
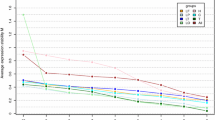
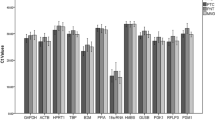
Pituitary surgery generates pituitary tissue for histology, immunohistochemistry, and molecular biological research. In the last decade, the pathogenesis of pituitary adenomas has been extensively studied in humans, and to a lesser degree in dogs, and tumor oncogenesis has been studied in knock-out mice, often by means of quantitative reversed-transcriptase PCR (RT-qPCR). A precondition of such analyses is that so-called reference genes are stably expressed regardless of changes in disease status or treatment. In this study, the expression of six frequently used reference genes, namely, tata box binding protein (tbp), tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide (ywhaz), hydroxymethylbilane synthase (hmbs), beta-2-microglobulin (b2m), succinate dehydrogenase complex subunit A (sdha), and glyceraldehyde 3 phosphate dehydrogenase 1 (gapdh), was studied in pituitary tissue (normal and adenoma) from three species (humans, mice, and dogs). The stability of expression of these reference genes differed between species and between healthy and diseased tissue within one species. Quantitative analysis based on a single reference gene that is assumed to be stably expressed might lead to wrong conclusions. This cross-species analysis clearly emphasizes the need to evaluate the expression stability of reference genes as a standard and integral aspect of study design and data analysis, in order to improve the validity of the conclusions drawn on the basis of quantitative molecular analyses.

Similar content being viewed by others
References
Asa SL, Ezzat S (2009) The pathogenesis of pituitary tumors. Annu Rev Pathol Mech Dis 4:97–126
Yeung CM, Chan CB, Leung PS, Cheng CHK (2006) Cells of the anterior pituitary. Int J Biochem Cell Biol 38:1441–1449
De Bruin C, Meij B, Kooistra H, Hanson J, Lamberts S, Hofland L (2009) Cushing’s disease in dogs and humans. Horm Res 71:140–143
Scheithauer BW, Gaffey TA, Lloyd RV, Sebo TJ, Kovacs KT, Horvath E, Yapcer Ö, Young WF Jr, Meyer FB, Kuroki T (2006) Pathobiology of pituitary adenomas and carcinomas. Neurosurgery 59:341–353
Beckers A (2010) Higher prevalence of clinically relevant pituitary adenomas confirmed. Clin Endocrinol (Oxf) 72:290–291
Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A (2006) High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab 91:4769–4775
Hanson JM, Teske E, Voorhout G, Galac S, Kooistra HS, Meij BP (2007) Prognostic factors for outcome after transsphenoidal hypophysectomy in dogs with pituitary-dependent hyperadrenocorticism. J Neurosurg 107:830–840
Willeberg P, Priester W (1982) Epidemiological aspects of clinical hyperadrenocorticism in dogs (canine Cushing’s syndrome). J Am Anim Hosp Assoc 18:717–724
Meij BP (2001) Hypophysectomy as a treatment for canine and feline Cushing’s disease. Vet Clin North Am Small Anim Pract 31:1015–1041
Melmed S (2011) Pathogenesis of pituitary tumors. Nature Rev Endocrin 7:257–266
Yu R, Melmed S (2010) Pathogenesis of pituitary tumors. Prog Brain Res 182:207–227
van Wijk PA, van Neck JW, Rijnberk A, Croughs RJM, Mol JA (1995) Proliferation of the murine corticotropic tumour cell line AtT20 is affected by hypophysiotrophic hormones, growth factors and glucocorticoids. Mol Cell Endocrinol 111:13–19
Hu N, Gutsmann A, Herbert D, Bradley A, Lee W, Lee E (1994) Heterozygous Rb-1 delta 20/ mice are predisposed to tumors of the pituitary gland with a nearly complete penetrance. Oncogene 9:1021–1027
Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA (2001) The sequence of the human genome. Science 291:1304–1351
Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, Kamal M, Clamp M, Chang JL, Kulbokas EJ, Zody MC (2005) Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438:803–819
Bustin SA, Beaulieu JF, Huggett J, Jaggi R, Kibenge FSB, Olsvik PA, Penning LC, Toegel S (2010) MIQE precis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol Biol 11:74
Dheda K, Huggett J, Chang J, Kim L, Bustin S, Johnson M, Rook G, Zumla A (2005) The implications of using an inappropriate reference gene for real-time reverse transcription PCR data normalization. Anal Biochem 344:141–143
Fukuoka H, Cooper O, Ben-Shlomo A, Mamelak A, Ren SG, Bruyette D, Melmed S (2011) EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J Clin Invest 121:4712–4721
Budry L, Balsalobre A, Gauthier Y, Khetchoumian K, L’Honoré A, Vallette S, Brue T, Figarella-Branger D, Meij B, Drouin J (2012) The selector gene Pax7 dictates alternate pituitary cell fates through its pioneer action on chromatin remodeling. Genes Dev 26:2299–2310
De Lima DS, Martins CS, Mc PB, Amaral FC, Colli LM, Saggioro FP, Neder L, Machado HR, Santos AR, Pinheiro DG (2012) SAGE analysis highlights the putative role of underexpression of Ribosomal Proteins in GH-secreting pituitary adenomas. Eur J Endocrinol 167:759–768
Chesnokova V, Zonis S, Wawrowsky K, Tani Y, Ben-Shlomo A, Ljubimov V, Mamelak A, Bannykh S, Melmed S (2012) Clusterin and FOXL2 act concordantly to regulate pituitary gonadotroph adenoma growth. Mol Endocrinol 26:2092–2103
Xie H, Cherrington BD, Meadows JD, Witham EA, Mellon PL (2013) Msx1 Homeodomain protein represses the αGSU and GnRH receptor genes during gonadotrope development. Mol Endocrinol 27:422–436
Newell-Price J, Trainer P, Besser M, Grossman A (1998) The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr Rev 19:647–672
Pivonello R, Ferone D, de Herder WW, Kros JM, De Caro ML, Arvigo M, Annunziato L, Lombardi G, Colao A, Hofland LJ, Lamberts SW (2004) Dopamine receptor expression and function in corticotroph pituitary tumors. J Clin Endocrinol Metab 89:2452–2462
Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA (1992) Effects of an Rb mutation in the mouse. Nature 359:295–300
Galac S, Kooistra H, Teske E, Rijnberk A (1997) Urinary corticoid/creatinine ratios in the differentiation between pituitary–dependent hyperadrenocorticism and hyperadrenocorticism due to adrenocortical tumour in the dog. Vet Q 19:17–20
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F, (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3, research0034
Andersen CL, Jensen JL, Ørntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245–5250
Ohl F, Jung M, Xu C, Stephan C, Rabien A, Burkhardt M, Nitsche A, Kristiansen G, Loening SA, Radonić A (2005) Gene expression studies in prostate cancer tissue: which reference gene should be selected for normalization? J Mol Med (Berl) 83:1014–1024
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
van Rijn SJ, Gremeaux L, Riemers FM, Brinkhof B, Vankelecom H, Penning LC, Meij BP (2012) Identification and characterisation of side population cells in the canine pituitary gland. Vet J 192:476–482
Korbonits M, Bustin SA, Kojima M, Jordan S, Adams EF, Lowe DG, Kangawa K, Grossman AB (2001) The expression of the growth hormone secretagogue receptor ligand ghrelin in normal and abnormal human pituitary and other neuroendocrine tumors. J Clin Endocrinol Metab 86:881–887
McCabe C, Khaira J, Boelaert K, Heaney A, Tannahill L, Hussain S, Mitchell R, Olliff J, Sheppard M, Franklyn J (2003) Expression of pituitary tumour transforming gene (PTTG) and fibroblast growth factor–2 (FGF–2) in human pituitary adenomas: relationships to clinical tumour behaviour. Clin Endocrinol (Oxf) 58:141–150
Lisowski P, Pierzchala M, Goscik J, Pareek CS, Zwierzchowski L (2008) Evaluation of reference genes for studies of gene expression in the bovine liver, kidney, pituitary, and thyroid. J Appl Genet 49:367–372
Jung M, Schaefer A, Steiner I, Kempkensteffen C, Stephan C, Erbersdobler A, Jung K (2010) Robust microRNA stability in degraded RNA preparations from human tissue and cell samples. Clin Chem 56:998–1006
Opitz L, Salinas-Riester G, Grade M, Jung K, Jo P, Emons G, Ghadimi BM, Beißbarth T, Gaedcke J (2010) Impact of RNA degradation on gene expression profiling. BMC Med Genomics 3:36
Acknowledgments
We thank Dr. A. de Bruin, Department of Pathobiology, Faculty of Veterinary Medicine, Utrecht University, Utrecht, the Netherlands, for generously donating the mice samples. We thank Jane Sykes for proofreading of the manuscript.
Disclosures
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Björn P. Meij and Louis C. Penning equally contributed to this work.
Rights and permissions
About this article
Cite this article
van Rijn, S.J., Riemers, F.M., van den Heuvel, D. et al. Expression Stability of Reference Genes for Quantitative RT-PCR of Healthy and Diseased Pituitary Tissue Samples Varies Between Humans, Mice, and Dogs. Mol Neurobiol 49, 893–899 (2014). https://doi.org/10.1007/s12035-013-8567-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8567-7