Abstract
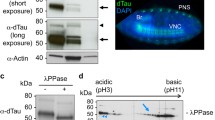
The identification of tau fragments generated by proteolysis in the neurons of AD patients and in neurofibrillary tangles encourages research on the toxicity of truncated tau. However, the detailed mechanism underlying the proteolysis-induced toxicity of tau is not clear and even controversial in some cases. In the present study, we used Drosophila as a model to evaluate the toxicity of a set of truncated tau fragments in vivo and found that the flies harboring C-terminal-truncated tau exhibited less toxicity due to the unstable characteristic of C-terminal-truncated tau fragments. Further study carried out in cultured Drosophila Kc cells revealed that C-terminal-truncated tau fragments degrade faster than full-length tau or N-terminal-truncated fragments. Collectively, our data implicate proteolysis of tau as an important pathway mediating tau degradation and neurotoxicity in vivo.






Similar content being viewed by others
References
Amadoro G, Ciotti MT, Costanzi M, Cestari V, Calissano P, Canu N (2006) NMDA receptor mediates tau-induced neurotoxicity by calpain and ERK/MAPK activation. Proc Natl Acad Sci U S A 103:2892–2897
Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT (1992) Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42:631–639
Basurto-Islas G, Luna-Munoz J, Guillozet-Bongaarts AL, Binder LI, Mena R, Garcia-Sierra F (2008) Accumulation of aspartic acid421- and glutamic acid391-cleaved tau in neurofibrillary tangles correlates with progression in Alzheimer disease. J Neuropathol Exp Neurol 67:470–483
Bednarski E, Lynch G (1996) Cytosolic proteolysis of tau by cathepsin D in hippocampus following suppression of cathepsins B and L. J Neurochem 67:1846–1855
Bugiani O, Murrell JR, Giaccone G et al (1999) Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in tau. J Neuropathol Exp Neurol 58:667–677
Canu N, Dus L, Barbato C et al (1998) Tau cleavage and dephosphorylation in cerebellar granule neurons undergoing apoptosis. J Neurosci 18:7061–7074
Cleveland DW, Hwo SY, Kirschner MW (1977) Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol 116:207–225
de Calignon A, Fox LM, Pitstick R et al (2010) Caspase activation precedes and leads to tangles. Nature 464:1201–1204
Delisle MB, Murrell JR, Richardson R et al (1999) A mutation at codon 279 (N279K) in exon 10 of the Tau gene causes a tauopathy with dementia and supranuclear palsy. Acta Neuropathol 98:62–77
DeVos SL, Goncharoff DK, Chen G et al (2013) Antisense reduction of tau in adult mice protects against seizures. J Neurosci 33:12887–12897
Dolan PJ, Johnson GV (2010) A caspase cleaved form of tau is preferentially degraded through the autophagy pathway. J Biol Chem 285:21978–21987
Eckermann K, Mocanu MM, Khlistunova I et al (2007) The beta-propensity of Tau determines aggregation and synaptic loss in inducible mouse models of tauopathy. J Biol Chem 282:31755–31765
Emery P (2007) Protein extraction from Drosophila heads. Methods Mol Biol (Clifton, NJ) 362:375–377
Fasulo L, Ugolini G, Visintin M et al (2000) The neuronal microtubule-associated protein tau is a substrate for caspase-3 and an effector of apoptosis. J Neurochem 75:624–633
Gamblin TC, Chen F, Zambrano A et al (2003) Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci U S A 100:10032–10037
Garg S, Timm T, Mandelkow EM, Mandelkow E, Wang Y (2011) Cleavage of tau by calpain in Alzheimer’s disease: the quest for the toxic 17 kD fragment. Neurobiol Aging 32:1–14
Giannakopoulos P, Herrmann FR, Bussiere T et al (2003) Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology 60:1495–1500
Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM (1986) Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem 261:6084–6089
Guillozet-Bongaarts AL, Cahill ME, Cryns VL, Reynolds MR, Berry RW, Binder LI (2006) Pseudophosphorylation of tau at serine 422 inhibits caspase cleavage: in vitro evidence and implications for tangle formation in vivo. J Neurochem 97:1005–1014
Hutton M, Lendon CL, Rizzu P et al (1998) Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393:702–705
Ihara Y (2001) PHF and PHF-like fibrils—cause or consequence? Neurobiol Aging 22:123–126
Jackson GR, Wiedau-Pazos M, Sang T-K et al (2002) Human wild-type tau interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron 34:509–519
Johnson GV, Jope RS, Binder LI (1989) Proteolysis of tau by calpain. Biochem Biophys Res Commun 163:1505–1511
Kenessey A, Nacharaju P, Ko LW, Yen SH (1997) Degradation of tau by lysosomal enzyme cathepsin D: implication for Alzheimer neurofibrillary degeneration. J Neurochem 69:2026–2038
Kramer JM, Staveley BE (2003) GAL4 causes developmental defects and apoptosis when expressed in the developing eye of Drosophila melanogaster. Genet Mol Res 2:43–47
Lee VM, Balin BJ, Otvos L Jr, Trojanowski JQ (1991) A68: a major subunit of paired helical filaments and derivatized forms of normal tau. Science 251:675–678
Lee VM, Goedert M, Trojanowski JQ (2001) Neurodegenerative tauopathies. Annu Rev Neurosci 24:1121–1159
Papanikolopoulou K, Skoulakis EM (2011) The power and richness of modelling tauopathies in Drosophila. Mol Neurobiol 44:122–133
Park SY, Ferreira A (2005) The generation of a 17 kDa neurotoxic fragment: an alternative mechanism by which tau mediates beta-amyloid-induced neurodegeneration. J Neurosci 25:5365–5375
Poorkaj P, Muma NA, Zhukareva V et al (2002) An R5L tau mutation in a subject with a progressive supranuclear palsy phenotype. Ann Neurol 52:511–516
Reinecke JB, DeVos SL, McGrath JP et al (2011) Implicating calpain in tau-mediated toxicity in vivo. PLoS ONE 6:e23865
Roberson ED, Scearce-Levie K, Palop JJ et al (2007) Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science 316:750–754
Santacruz K, Lewis J, Spires T et al (2005) Tau suppression in a neurodegenerative mouse model improves memory function. Science 309:476–481
Shulman JM, Imboywa S, Giagtzoglou N et al (2013) Functional screening in Drosophila identifies Alzheimer’s disease susceptibility genes and implicates tau-mediated mechanisms. Hum Mol Genet 23:870–877
Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B (1998) Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci U S A 95:7737–7741
Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW (1975) A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A 72:1858–1862
Witman GB, Cleveland DW, Weingarten MD, Kirschner MW (1976) Tubulin requires tau for growth onto microtubule initiating sites. Proc Natl Acad Sci U S A 73:4070–4074
Wittmann CW, Wszolek MF, Shulman JM et al (2001) Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 293:711–714
Wolff T (2011) Preparation of Drosophila eye specimens for scanning electron microscopy. Cold Spring Harb Protoc 2011:1383–1385
Wood JG, Mirra SS, Pollock NJ, Binder LI (1986) Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau). Proc Natl Acad Sci U S A 83:4040–4043
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (NSFC) (31170737), National Major Scientific and Technological Special Project for “Significant New Drugs Development” (2013ZX09103001-019), and Beijing New Medical Discipline Based Group (100270569).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geng, J., Xia, L., Li, W. et al. The C-Terminus of Tau Protein Plays an Important Role in Its Stability and Toxicity. J Mol Neurosci 55, 251–259 (2015). https://doi.org/10.1007/s12031-014-0314-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-014-0314-7