Abstract
Background/Objectives
We recently developed techniques to monitor intraspinal pressure (ISP) and spinal cord perfusion pressure (SCPP) from the injury site to compute the optimum SCPP (SCPPopt) in patients with acute traumatic spinal cord injury (TSCI). We hypothesized that ISP and SCPPopt can be predicted using clinical factors instead of ISP monitoring.
Methods
Sixty-four TSCI patients, grades A–C (American spinal injuries association Impairment Scale, AIS), were analyzed. For 24 h after surgery, we monitored ISP and SCPP and computed SCPPopt (SCPP that optimizes pressure reactivity). We studied how well 28 factors correlate with mean ISP or SCPPopt including 7 patient-related, 3 injury-related, 6 management-related, and 12 preoperative MRI-related factors.
Results
All patients underwent surgery to restore normal spinal alignment within 72 h of injury. Fifty-one percentage had U-shaped sPRx versus SCPP curves, thus allowing SCPPopt to be computed. Thirteen percentage, all AIS grade A or B, had no U-shaped sPRx versus SCPP curves. Thirty-six percentage (22/64) had U-shaped sPRx versus SCPP curves, but the SCPP did not reach the minimum of the curve, and thus, an exact SCPPopt could not be calculated. In total 5/28 factors were associated with lower ISP: older age, excess alcohol consumption, nonconus medullaris injury, expansion duroplasty, and less intraoperative bleeding. In a multivariate logistic regression model, these 5 factors predicted ISP as normal or high with 73% accuracy. Only 2/28 factors correlated with lower SCPPopt: higher mean ISP and conus medullaris injury. In an ordinal multivariate logistic regression model, these 2 factors predicted SCPPopt as low, medium–low, medium–high, or high with only 42% accuracy. No MRI factors correlated with ISP or SCPPopt.
Conclusions
Elevated ISP can be predicted by clinical factors. Modifiable factors that may lower ISP are: reducing surgical bleeding and performing expansion duroplasty. No factors accurately predict SCPPopt; thus, invasive monitoring remains the only way to estimate SCPPopt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic spinal cord injury (TSCI) is a catastrophic condition: Over a third of patients do not recover sensation or voluntary movement below the injury [1]. No treatment has been proved to improve outcome [2], and therefore, patient management remains variable [3]. To guide the management of patients with acute, severe TSCI in the intensive care unit, we introduced intraspinal pressure (ISP) monitoring from the injury site [4, 5]. The technique is safe [6] and allows us to compute the spinal cord perfusion pressure (SCPP) as mean arterial pressure (MAP) minus ISP and the spinal pressure reactivity index (sPRx) as the running correlation coefficient between ISP and MAP. sPRx measures the pressure reactivity of the vascular bed, i.e., autoregulation. sPRx ≤ 0 indicates intact vascular pressure reactivity, whereas sPRx > 0 indicates deficient vascular pressure reactivity. At low SCPP (hypo-perfusion) and high SCPP (hyper-perfusion), autoregulation is impaired (i.e., high sPRx) [7,8,9]. Thus, the sPRx versus SCPP plot is U-shaped and the optimum SCPP (SCPPopt) is the minimum of the curve. The U-shaped curve is not always present and may vary throughout the period of monitoring; when computed in patients with head injury or spinal cord injury, a U-shaped sPRx versus SCPP relation is present approximately 50% of the time. ISP, SCPP, SCPPopt, and sPRx for TSCI are, respectively, analogous to intracranial pressure (ICP), cerebral perfusion pressure (CPP), optimum CPP (CPPopt), and cerebrovascular pressure reactivity (PRx) for traumatic brain injury (TBI) [10].
TSCI patients with mean SCPP ≈ SCPPopt more often improve their AIS (American spinal injuries association Impairment Scale) grade than patients with SCPP very different from SCPPopt [8]. Thus, targeting the SCPPopt could form the basis for individualizing the treatment of TSCI in a physiologically meaningful manner. When compared with the current guideline of maintaining MAP at 85–95 mmHg in all TSCI patients [11], the concept of individualized management, based on SCPPopt, represents a paradigm shift. A universal MAP target is inadequate because TSCI patients have different ISPs and because the SCPPopt varies widely between patients [4, 5, 8]. The aim here is to identify clinical and magnetic resonance imaging (MRI) features for predicting ISP and SCPPopt in each TSCI patient. The ability to estimate ISP and SCPPopt noninvasively would be a major advance by allowing doctors to calculate a target MAP for each TSCI patient without ISP monitoring.
Materials and Methods
Institutional Approvals
Patients were recruited as part of the Injured Spinal Cord Pressure Evaluation (ISCoPE) study (ClinicalTrials.gov as NCT02721615). Approvals for ISCoPE were obtained from the St George’s, University of London Joint Research Office and the National Research Ethics Service London–St Giles Committee (No. 10/H0807/23).
Patient Recruitment
We include all (consecutive) TSCI patients recruited between October 2010 and December 2017. Inclusion criteria are: (1) severe TSCI defined as AIS grade A, B, or C; (2) age 18–70 years; (3) timing between TSCI and surgery within 72 h. Exclusion criteria are: (1) patient unable to consent; (2) other major comorbidities; (3) penetrating TSCI.
Surgery and ISP Monitoring
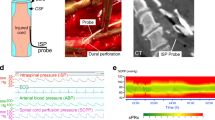
Surgical decompression and spinal instrumentation were performed based on patient requirements and surgeon preference. Some patients also had duroplasty as described [12]. During surgery, an ISP probe (Codman Microsensor Transducer®, Depuy Synthes, Leeds, UK) was placed intradurally on the surface of the injured cord at the site of maximal cord swelling. The dural opening was sutured and supplemented with fibrin glue (Tisseel®, Baxter, UK). The ISP probe was connected to a Codman ICP box linked via a ML221 amplifier to a PowerLab running LabChart v.7.3.5 (AD Instruments, Oxford, UK). Arterial blood pressure was recorded from a radial artery catheter connected to the Philips Intellivue MX800 bedside monitoring system (Philips, Guildford, UK), and in turn connected to the PowerLab system. ISP and arterial blood pressure signals were sampled at 1 kHz for 24 h after surgery. When the spinal cord is swollen and compressed against the dura, the subdural pressure (i.e., ISP) and intraparenchymal pressure at the injury site are the same. In this case, ISP is higher than cerebrospinal fluid (CSF) pressure above or below the injury site—details are given elsewhere [5, 6, 13,14,15].
ISP, SCPP, and SCPPopt
We processed signals using ICM + to compute SCPP (as ISP minus MAP) and sPRx (as the running correlation coefficient between ISP and MAP). Microsoft Excel was used to produce sPRx versus SCPP plots and estimate the SCPPopt as the minimum of the curve. We used the ISP and MAP signals recorded over the 24 h after surgery to compute the average ISP, the average SCPP, as well as the overall SCPPopt. The ISP monitoring setup is illustrated in Fig. 1. We chose the first 24 h of ISP monitoring, because these are the closest to the timing of the preoperative MRI that was used to determine the imaging features. Also, because ISP was monitored for different periods in different patients, we only included the first 24 h to standardize the duration of monitoring. We arbitrarily defined ISP as high if > 20 mmHg and normal if ≤ 20 mmHg based on our previous ISP analysis in patients with TSCI versus controls [5]. The study protocol intentionally did not set ISP or SCPP targets, and the ethical approval was to monitor ISP and SCPP, but not use these values to alter management. This arrangement allowed us to study the injured cord over a wide range of ISPs and SCPPs. MAP was managed at the discretion of the consultant intensive care physician. In general, MAP 80–100 mmHg was maintained for a week after TSCI.
ISP monitoring technique. a Preoperative MRI of a 37-year-old male patient with TSCI AIS grade C at C3/4. b Postoperative CT of same patient showing C3/4 anterior cervical cage, posterior C3/4 laminectomies, and ISP probe (circled). c MAP, ISP SCPP signals. d sPRx versus SCPP plot. Minimum is SCPPopt
Clinical Features
A total of 28 factors were collected for each patient as follows:
-
1.
Age,
-
2.
Sex,
-
3.
Smoking,
-
4.
Hypertension,
-
5.
Diabetes,
-
6.
History of excess alcohol consumption,
-
7.
Body mass index,
-
8.
Level of injury, i.e., cervical/thoracic/conus,
-
9.
AIS grade on admission,
-
10.
Primary survey MAP,
-
11.
Hours from injury to surgery,
-
12.
Duration of surgery,
-
13.
Intraoperative blood loss,
-
14.
Extent of decompression i.e., spinal alignment/spinal alignment + laminectomy/spinal alignment + laminectomy + duroplasty,
-
15.
Surgical approach, i.e., posterior/anterior + posterior,
-
16.
Mean MAP during surgery,
-
17.
Epidural hematoma,
-
18.
Intraparenchymal hematoma,
-
19.
Cord transection,
-
20.
Number of sagittal inter-vertebral levels with no CSF signal [16],
-
21.
Injury level average spinal cord occupation rate: SCOR [16],
-
21.
Brain and spinal injury center score BASIC [17],
-
23.
Sagittal grade 1–4 [18],
-
24.
Longitudinal extent of T2 signal abnormality [19],
- 25.
-
26.
Maximum canal compromise MCC [20],
-
27.
Average normal cord in injured cord [22],
-
28.
Average % cord signal change at injured level [16].
Detailed definitions of the 28 factors are given in the Supplement. Data regarding the 28 factors were collected without knowledge of the ISP, SCPP, and SCPPopt data.
Statistical Analysis
Analyses were carried out with XLStat Biomed (v.18.07, Addinsoft, New York, USA). For univariate analyses, we used Mann–Whitney U or Wilcoxon rank sum with post hoc Dunn to investigate differences in ISP or SCPPopt between the factors. In the multivariate logistic regression analysis for ISP, we chose the best model based on likelihood by including factors with P < 0.1 and removing factors already in the model if P > 0.2 when a new factor is added. In the multivariate ordinal logistic regression analysis for SCPPopt, we chose a stepwise forward model based on the Wald criterion; the model enters factors with P < 0.1 and removes factors already in the model if P > 0.2 when a new factor is added. Correlations between two factors were quantified with the Spearman’s rank correlation coefficient “ρ”.
Results
Patient Characteristics
Table 1 provides information on the demographic characteristics of the 64 patients. Most are young, with 80% < 60 years old. Males outnumber females by 3.2:1. Cervical TSCIs are more common than thoracic or conus at 52 versus 34 versus 14%. Most patients had neurologically complete TSCI on admission, i.e., 67% were ASIA grade A. Fifty-one percentage (34/64) had U-shaped sPRx versus SCPP curves for the first 24 h after surgery, thus allowing SCPPopt to be computed. Mean SCPPopt for all patients was 74 mmHg (range 48–103). Thirteen percentage (8/64), all AIS grade A or B, had no U-shaped sPRx versus SCPP curve for the first 24 h after surgery. The remaining 36% (22/64) patients appeared to have U-shaped sPRx versus SCPP curves, but the range of SCPPs did not reach the minimum, and thus, exact SCPPopt could not be calculated.
Predictors of Mean ISP
In univariate analysis, only 5/28 characteristics that were examined correlated with ISP. The 5 characteristics that were associated with higher mean ISP are no excess alcohol consumption, lower cord injury, not having a duroplasty, younger age, and more intraoperative blood loss (Fig. 2). Interestingly, none of the 12 MRI characteristics that we investigated correlated with mean ISP. A multivariate logistic regression model based on these 5 characteristics could classify ISP as normal (< 20 mmHg) versus high (≥ 20 mmHg) with 73% accuracy (Table 2). In this multivariate model, which takes into account interactions between the characteristics, no excess alcohol consumption (odds ratio 9.6, 95% CI 1.2–74.9), lower injury level (odds ratio cervical to thoracic to conus 3.3, 95% CI 1.1–10.3), no expansion duroplasty (odds ratio 3.2, 95% CI 1.1–12.3), and younger age (odds ratio per decade 1.5, 95% CI 1.1–2.4) remained significant (each at P < 0.05) predictors of high ISP. Intraoperative blood loss was no longer significant because of collinearity with the level of injury (ρ = 0.59), i.e., more blood loss when operating lower down the spine.
Factors that correlate with mean ISP. a Level of spinal cord injury (cervical, thoracic, conus). b Age group in years (< 30, 30 – 40, 40 – 50, 50 – 60, > 60). c Extent of decompression (Spinal Alignment, Spinal Alignment + Laminectomy, Spinal Alignment + Laminectomy + Duroplasty). d Intraoperative blood loss as % of total blood volume (< 15%, 15 – 30%, 30 – 40%, > 40%). e Excess alcohol consumption. Box plots show median, upper and lower quartiles, minimum and maximum. Gray trend line. P < 0.05*, 0.005#
Predictors of Mean 24-h SCPPopt
Each patient was assigned to a SCPPopt group as low (< 60 mmHg), low–medium (60–70 mmHg), high–medium (70–80 mmHg), or high (> 80 mmHg). This allowed us to analyze 51 patients in total, by including not only the 34 patients who had exact SCPPopt, but also 8 patients with SCPPopt > 80 mmHg and 9 patients with SCPPopt < 60 mmHg. In univariate analysis, only 2/29 factors that were examined correlated with lower mean SCPPopt: higher mean 24-h ISP and lower spinal level of injury (Fig. 3a, b). These two factors (level of spinal cord injury and mean 24-h ISP) were correlated (ρ = 0.49). Interestingly, again none of the 12 MRI factors correlated with mean SCPPopt. An ordinal logistic regression model based on these two factors could classify SCPPopt as low, low–medium, high–medium, or high with only 42% accuracy (Table 3).
Discussion
In this study we have shown that after a TSCI clinical features may be used to predict whether ISP is elevated, but clinical features cannot accurately predict SCPPopt. Risk factors for high ISP include younger age, conus injury, no excessive alcohol intake, no duroplasty, and large intraoperative blood loss. We also showed that patients with conus injuries and those with high ISP have low SCPPopt and that some patients with severe TSCI have no computable SCPPopt. There were no MRI features associated with ISP or SCPPopt.
Clinical features that correlate with ISP may shed light on the pathophysiology of TSCI. The factor that protects the most from elevated ISP is history of excess alcohol consumption documented in the patient notes. The underlying mechanism may be cord atrophy, i.e., more CSF space to accommodate the swollen cord, analogous to TBI where alcohol-related brain atrophy might protect from elevated ICP [23,24,25]. Expansion duroplasty and the level of injury have comparable protective effect on ISP. We already showed that duroplasty significantly reduces ISP after TSCI by enlarging the CSF space around the injured cord [12]. The higher ISP with conus medullaris injuries is likely related to the reduced CSF space around the conus medullaris compared with the larger CSF space higher up the spine [26]. The next factor that correlates with lower ISP is older age, perhaps because of cord atrophy in older patients, analogous to age-related brain atrophy [27, 28]. Higher ISP in patients with more intraoperative bleeding may be due to the level of TSCI rather than the bleeding per se, based on the positive correlation between surgical blood loss and level of TSCI. Alternatively, intraoperative fluid resuscitation may play a role, with larger fluid volumes administered exacerbating the edema at the injury site. The unifying theme here is that ISP after TSCI is determined by the relative dimensions of the cord at the injury site compared with the surrounding CSF space. Larger CSF space (cord atrophy, cervicothoracic injuries, duroplasty) is associated with lower ISP.
We showed that SCPPopt is lower in patients with higher ISP or conus TSCI. Though high SCPP is beneficial by reducing ischemia, these observations suggest that as the spinal cord becomes more compressed, high SCPP may become detrimental. The mechanism might be blood pressure induced local steal, a phenomenon whereby increasing the MAP when the cord is swollen causes a “paradoxical” decrease in blood flow at the injury site [29]. Knowledge of SCPPopt is clinically important to reduce secondary cord damage by hypo- or hyper-perfusion. Since clinical features do not accurately predict SCPPopt, invasive ISP monitoring remains the only way of determining SCPPopt. A recent study showed that SCPP can be calculated by monitoring CSF pressure using a lumbar catheter and that SCPP measured in this way better correlates with outcome than MAP [30]. Monitoring CSF pressure is less invasive than monitoring ISP. However, the relation between SCPP measured by ISP monitoring and by lumbar drain is unclear. It is also unclear whether CSF pressure can be used to compute sPRx and SCPPopt.
An unanticipated finding is that no MRI features correlated with ISP or SCPPopt. This may be because we used preoperative MRIs, performed when the spinal anatomy was abnormal. Surgery restores the normal spinal alignment, thus causing major anatomical changes at the injury site. MRI performed immediately after surgery may thus be more informative for estimating ISP and SCPPopt than preoperative MRIs. Earlier studies support the notion preoperative MRI features only weakly correlate with outcome, and in general, postoperative MRIs are more informative [31,32,33]. The take-home message is that preoperative MRIs cannot be relied upon to estimate the degree of cord compression or the optimal MAP to target after surgery.
Our study has limitations. ISP monitoring is an invasive technique, and currently, ours is the only unit that performs such monitoring in acute TSCI. Corroboration of our results in other centers is essential. Thus, the concepts of ISP, SCPP, SCPPopt, and sPRx remain theoretical and a randomized study to assess outcomes of patients managed according to their individual injury site physiology rather than applying universal MAP targets is necessary before ISP monitoring becomes definitively recommended. Finally, our cohort of 64 patients is relatively small, and thus, weaker associations between the 28 clinical features investigated here ISP and SCPPopt might be missed.
Conclusions
To conclude, our study supports the notion that ISP is determined by the size of the CSF space surrounding the injured cord. In other words, after TSCI, the cord swells and is compressed by the dura [34], thus, factors that increase the surrounding CSF space such as expansion duraplasty may be beneficial [12]. Based on our data, we urge caution when increasing the MAP, without knowing the SCPPopt, in patients whose injured cord is very compressed, i.e., high ISP. The individualized U-shaped curves suggest that SCPP > SCPPopt may be detrimental. Hyper-perfusing injured cord is associated with more deranged injury site metabolism [35]. In TBI patients, hyper-perfusion of the brain is associated with worse neurological outcome [36], although this finding has not yet been replicated in TSCI (due to insufficient number of TSCI patients exceeding the SCPPopt), the same trend may apply [8]. To date, the only way to avoid hyper-perfusing the injured cord is by determining the SCPPopt by ISP monitoring.
At present there is no consensus about the correct paradigm for managing acute TSCI [2, 3, 37, 38] although there is growing evidence and support for a randomized trial to assess whether novel treatments such as invasive ISP monitoring, SCPP optimization, and expansion duroplasty improve outcomes [39].
References
National Spinal Cord Injury Statistical Center. Spinal cord injury facts and figures at a glance. J Spinal Cord Med. 2014;37:659–60.
van Middendorp JJ, Goss B, Urquhart S, Atresh S, Williams RP, Schuetz M. Diagnosis and prognosis of traumatic spinal cord injury. Glob Spine J. 2011;1:1–8.
Werndle MC, Zoumprouli A, Sedgwick P, Papadopoulos MC. Variability in the treatment of acute spinal cord injury in the United Kingdom: results of a national survey. J Neurotrauma. 2012;29:880–8.
Varsos GV, Werndle MC, Czosnyka ZH, et al. Intraspinal pressure and spinal cord perfusion pressure after spinal cord injury: an observational study. J Neurosurg Spine. 2015;23:763–71.
Werndle MC, Saadoun S, Phang I, et al. Monitoring of spinal cord perfusion pressure in acute spinal cord injury: initial findings of the injured spinal cord pressure evaluation study. Crit Care Med. 2014;42:646–55.
Phang I, Zoumprouli A, Saadoun S, Papadopoulos MC. Safety profile and probe placement accuracy of intraspinal pressure monitoring for traumatic spinal cord injury: injured Spinal Cord Pressure Evaluation study. J Neurosurg Spine. 2016;25:398–405.
Aries MJ, Czosnyka M, Budohoski KP, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40:2456–63.
Chen S, Smielewski P, Czosnyka M, Papadopoulos MC, Saadoun S. Continuous monitoring and visualization of optimum spinal cord perfusion pressure in patients with acute cord injury. J Neurotrauma. 2017;34:2941–9.
Steiner LA, Czosnyka M, Piechnik SK, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30:733–8.
Needham E, McFadyen C, Newcombe V, Synnot AJ, Czosnyka M, Menon D. Cerebral perfusion pressure targets individualized to pressure-reactivity index in moderate to severe traumatic brain injury: a systematic review. J Neurotrauma. 2017;34:963–70.
Walters BC, Hadley MN, Hurlbert RJ, et al. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery. 2013;60(Suppl 1):82–91.
Phang I, Werndle MC, Saadoun S, et al. Expansion duroplasty improves intraspinal pressure, spinal cord perfusion pressure, and vascular pressure reactivity index in patients with traumatic spinal cord injury: injured spinal cord pressure evaluation study. J Neurotrauma. 2015;32:865–74.
Papadopoulos MC. Intrathecal pressure after spinal cord injury. Neurosurgery. 2015;77:E500.
Phang I, Papadopoulos MC. Intraspinal pressure monitoring in a patient with spinal cord injury reveals different intradural compartments: injured Spinal Cord Pressure Evaluation (ISCoPE) Study. Neurocrit Care. 2015;23:414–8.
Werndle MC, Saadoun S, Phang I, et al. Measurement of intraspinal pressure after spinal cord injury: technical note from the injured spinal cord pressure evaluation study. Acta Neurochir Suppl. 2016;122:323–8.
Kato F, Yukawa Y, Suda K, Yamagata M, Ueta T. Normal morphology, age-related changes and abnormal findings of the cervical spine. Part II: magnetic resonance imaging of over 1,200 asymptomatic subjects. Eur Spine J. 2012;21:1499–507.
Talbott JF, Whetstone WD, Readdy WJ, et al. The Brain and Spinal Injury Center score: a novel, simple, and reproducible method for assessing the severity of acute cervical spinal cord injury with axial T2-weighted MRI findings. J Neurosurg Spine. 2015;23:495–504.
Bondurant FJ, Cotler HB, Kulkarni MV, McArdle CB, Harris JH. Acute spinal cord injury. A study using physical examination and magnetic resonance imaging. Spine (Phila PA 1976). 1990;15:161–8.
Biering-Sørensen F, Alai S, Anderson K, et al. Common data elements for spinal cord injury clinical research: a National Institute for Neurological Disorders and Stroke project. Spinal Cord. 2015;53:265–77.
Fehlings MG, Rao SC, Tator CH, et al. The optimal radiologic method for assessing spinal canal compromise and cord compression in patients with cervical spinal cord injury. Part II: results of a multicenter study. Spine (Phila Pa 1976). 1999;24:605–13.
Rao SC, Fehlings MG. The optimal radiologic method for assessing spinal canal compromise and cord compression in patients with cervical spinal cord injury. Part I: an evidence-based analysis of the published literature. Spine (Phila Pa 1976). 1999;24:598–604.
Rüegg TB, Wicki AG, Aebli N, Wisianowsky C, Krebs J. The diagnostic value of magnetic resonance imaging measurements for assessing cervical spinal canal stenosis. J Neurosurg Spine. 2015;22:230–6.
Harper C, Kril J. Brain atrophy in chronic alcoholic patients: a quantitative pathological study. J Neurol Neurosurg Psychiatry. 1985;48:211–7.
García-Valdecasas-Campelo E, González-Reimers E, Santolaria-Fernández F, et al. Brain atrophy in alcoholics: relationship with alcohol intake; liver disease; nutritional status, and inflammation. Alcohol Alcohol. 2007;42:533–8.
Chesnut R, Videtta W, Vespa P, Le Roux P. Monitoring PitIMCCoM. Intracranial pressure monitoring: fundamental considerations and rationale for monitoring. Neurocrit Care. 2014;21(Suppl 2):S64–84.
Park JW, Bae SK, Huh J. Distance from Dura mater to spinal cord at the thoracic vertebral level: an introductory study on local subdural geometry for thoracic epidural block. J Int Med Res. 2016;44:950–6.
Farin A, Deutsch R, Biegon A, Marshall LF. Sex-related differences in patients with severe head injury: greater susceptibility to brain swelling in female patients 50 years of age and younger. J Neurosurg. 2003;98:32–6.
Czosnyka M, Balestreri M, Steiner L, et al. Age, intracranial pressure, autoregulation, and outcome after brain trauma. J Neurosurg. 2005;102:450–4.
Gallagher MJ, Hogg FRA, Zoumprouli A, Papadopoulos MC, Saadoun S. Spinal cord blood flow in patients with acute spinal cord injuries. J Neurotrauma (in press).
Squair JW, Belanger LM, Tsang A, et al. Spinal cord perfusion pressure predicts neurologic recovery in acute spinal cord injury. Neurology. 2017;89:1660–7.
Huber E, Lachappelle P, Sutter R, Curt A, Freund P. Are midsagittal tissue bridges predictive of outcome after cervical spinal cord injury? Ann Neurol. 2017;81:740–8.
Freund P, Weiskopf N, Ashburner J, et al. MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury: a prospective longitudinal study. Lancet Neurol. 2013;12:873–81.
Skeers P, Battistuzzo CR, Clark JM, Bernard S, Freeman BJC, Batchelor PE. Acute Thoracolumbar Spinal Cord Injury: relationship of cord compression to neurological outcome. J Bone Joint Surg Am. 2018;100:305–15.
Saadoun S, Werndle MC, Lopez de Heredia L, Papadopoulos MC. The dura causes spinal cord compression after spinal cord injury. Br J Neurosurg. 2016;30:582–4.
Phang I, Zoumprouli A, Papadopoulos MC, Saadoun S. Microdialysis to optimize cord perfusion and drug delivery in spinal cord injury. Ann Neurol. 2016;80:522–31.
Aries MJ, Czosnyka M, Budohoski KP, et al. Continuous monitoring of cerebrovascular reactivity using pulse waveform of intracranial pressure. Neurocrit Care. 2012;17:67–76.
Fehlings MG, Vaccaro A, Wilson JR, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS ONE. 2012;7:e32037.
van Middendorp JJ, Hosman AJ, Doi SA. The effects of the timing of spinal surgery after traumatic spinal cord injury: a systematic review and meta-analysis. J Neurotrauma. 2013;30:1781–94.
Tykocki T, Poniatowski Ł, Czyż M, Koziara M, Wynne-Jones G. Intraspinal pressure monitoring and extensive duroplasty in the acute phase of traumatic spinal cord injury: a systematic review. World Neurosurg. 2017;105:145–52.
Acknowledgements
We thank the neurosurgeons at St. George’s Hospital, King’s College Hospital and Hurstwood Park Neurological Centre as well as the spinal orthopedic surgeons at St. George’s Hospital who helped recruit patients. The neuroanaesthetic, neurointensive care, and operating theater staff at St. George’s Hospital helped with data collection.
Funding
This work was supported by grants awarded to MCP and SS from the Wings for Life Spinal Cord Research Foundation and to MCP from the Neurosciences Research Foundation (Fletcher Fund). FRAH is a UK Stem Cell Foundation and Royal College of Surgeons of England research fellow. MJG is supported by the Neurosciences Research Foundation (Fletcher Fund) and the London Deanery. Supported by the Wings for Life Spinal Cord Research Foundation, the Fletcher Fund, the Neurosciences Research Foundation and the London Deanery.
Author information
Authors and Affiliations
Contributions
FRAH collected and analyzed data and helped write the manuscript and produce the figures. MJG collected and analyzed data, and SC helped with data analysis. AZ anaesthetized the patients and helped with data collection and contributed key ideas to the manuscript. MCP operated on all the patients, helped with data analysis, and co-wrote the manuscript. SS designed the study, did data analysis, produced the figures, co-wrote the manuscript, and provided overall supervision for the project.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed here were in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Co-senior authors: Marios C. Papadopoulos and Samira Saadoun.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hogg, F.R.A., Gallagher, M.J., Chen, S. et al. Predictors of Intraspinal Pressure and Optimal Cord Perfusion Pressure After Traumatic Spinal Cord Injury. Neurocrit Care 30, 421–428 (2019). https://doi.org/10.1007/s12028-018-0616-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-018-0616-7