Abstract
Myocardial infarction (MI), triggered by blockage of a coronary artery, remains the most common cause of death worldwide. After MI, the capability of providing sufficient blood and oxygen significantly decreases in the heart. This event leads to depletion of oxygen from cardiac tissue and consequently leads to massive cardiac cell death due to hypoxemia. Over the past few decades, many studies have been carried out to discover acceptable approaches to treat MI. However, very few have addressed the crucial role of efficient oxygen delivery to the injured heart. Thus, various strategies were developed to increase the delivery of oxygen to cardiac tissue and improve its function. Here, we have given an overall discussion of the oxygen delivery mechanisms and how the current technologies are employed to treat patients suffering from MI, including a comprehensive view on three major technical approaches such as oxygen therapy, hemoglobin-based oxygen carriers (HBOCs), and oxygen-releasing biomaterials (ORBs). Although oxygen therapy and HBOCs have shown promising results in several animal and clinical studies, they still have a few drawbacks which limit their effectiveness. More recent studies have investigated the efficacy of ORBs which may play a key role in the future of oxygenation of cardiac tissue. In addition, a summary of conducted studies under each approach and the remaining challenges of these methods are discussed.

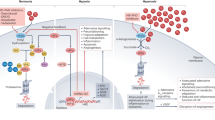
Reprinted with permission from [59] under the Creative Commons CC-BY-NC-ND 4.0 license

Reprinted with permission from [59] under the Creative Commons CC-BY-NC-ND 4.0 license

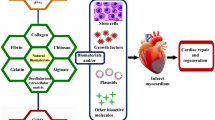
Reprinted with permission from [84] under the Creative Commons Attribution License


Reprinted with permission from [86] under a Creative Commons Attribution 4.0 International License

Similar content being viewed by others
Abbreviations
- MI:
-
Myocardial infarction
- HBOCs:
-
Hemoglobin-based oxygen carriers
- ORBs:
-
Oxygen-releasing biomaterials
- IGF-1:
-
Insulin-like growth factor
- PDGF:
-
Platelet-derived growth factor
- ATP:
-
Adenosine triphosphate
- AMI:
-
Acute myocardial infarction
- FIO2 :
-
Fraction of inspired oxygen
- HBO:
-
Hyperbaric oxygen
- MSCs:
-
Mesenchymal stem cells
- IR:
-
Ischemia–reperfusion
- WR/WLV:
-
Weight of risk/weight of left ventricle
- WI/WLV:
-
Weight of infarct/weight of left ventricle
- ROS:
-
Reactive oxygen species
- PolyPHb:
-
Polymerized human placenta hemoglobin
- PDMS:
-
Polydimethylsiloxane
- 3D:
-
Three-dimensional
- PFO:
-
Perfluorooctane
- hPDCs:
-
Human periosteal cells
- SPO:
-
Sodium percarbonate
- CPO:
-
Calcium peroxide
- T1D:
-
Type 1 diabetes
- β cells:
-
Beta cells
- CSPs:
-
Cardiac side population cells
- CPO-GelMA:
-
Calcium peroxide laden gelatin methacryloyle
- PFC:
-
Perfluorocarbon
References
Ibanez, B., James, S., Agewall, S., Antunes, M. J., Bucciarelli-Ducci, C., Bueno, H., Caforio, A. L., Crea, F., Goudevenos, J. A., Halvorsen, S., & Hindricks, G. (2018). 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). European Heart Journal, 39(2), 119–177.
Roth, G. A., Johnson, C., Abajobir, A., Abd-Allah, F., Abera, S. F., Abyu, G., Ahmed, M., Aksut, B., Alam, T., Alam, K., & Alla, F. (2017). Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. Journal of the American College of Cardiology, 70(1), 1–25.
Kemp, C. D., & Conte, J. V. (2012). The pathophysiology of heart failure. Cardiovascular Pathology, 21(5), 365–371.
Pascual-Gil, S., Garbayo, E., Díaz-Herráez, P., Prosper, F., & Blanco-Prieto, M. J. (2015). Heart regeneration after myocardial infarction using synthetic biomaterials. Journal of Controlled Release, 203, 23–38.
Olivetti, G., Abbi, R., Quaini, F., Kajstura, J., Cheng, W., Nitahara, J. A., Quaini, E., Di Loreto, C., Beltrami, C. A., Krajewski, S., & Reed, J. C. (1997). Apoptosis in the failing human heart. New England Journal of Medicine, 336(16), 1131–1141.
Park, A.-M., & Suzuki, Y. J. (2007). Effects of intermittent hypoxia on oxidative stress-induced myocardial damage in mice. Journal of Applied Physiology, 102(5), 1806–1814.
Wang, R., Yang, Q., Wang, X., Wang, W., Li, J., Zhu, J., Liu, X., Liu, J., & Du, J. (2016). FoxO3α-mediated autophagy contributes to apoptosis in cardiac microvascular endothelial cells under hypoxia. Microvascular research, 104, 23–31.
Zhong, Z., Hu, J. Q., Wu, X. D., Sun, Y., & Jiang, J. (2016). Anti-apoptotic effects of myocardin-related transcription factor-A on rat cardiomyocytes following hypoxia-induced injury. Canadian journal of physiology and pharmacology, 94(4), 379–387.
Etzion, S., Kedes, L. H., Kloner, R. A., & Leor, J. (2001). Myocardial regeneration. American journal of Cardiovascular Drugs, 1(4), 233–244.
Reboucas, J. S., Santos-Magalhaes, N. S., & Formiga, F. R. (2016). Cardiac regeneration using growth factors: Advances and challenges. Arquivos Brasileiros de Cardiologia, 107(3), 271–275.
Huang, K., Hu, S., & Cheng, K. (2019). A new era of cardiac cell therapy: Opportunities and challenges. Advanced Healthcare Materials, 8(2), 1801011.
Jawad, H., Ali, N. N., Lyon, A. R., Chen, Q. Z., Harding, S. E., & Boccaccini, A. R. (2007). Myocardial tissue engineering: A review. Journal of Tissue Engineering and Regenerative Medicine, 1(5), 327–342.
Tang, Y. L., Zhu, W., Cheng, M., Chen, L., Zhang, J., Sun, T., Kishore, R., Phillips, M. I., Losordo, D. W., & Qin, G. (2009). Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circulation Research, 104(10), 1209–1216.
Costanzo, M. R., Augustine, S., Bourge, R., Bristow, M., O’Connell, J. B., Driscoll, D., & Rose, E. (1995). Selection and treatment of candidates for heart transplantation: A statement for health professionals from the Committee on Heart Failure and Cardiac Transplantation of the Council on Clinical Cardiology, American Heart Association. Circulation, 92(12), 3593–3612.
Michaels, A. D., & Chatterjee, K. (2002). Angioplasty versus bypass surgery for coronary artery disease. Circulation, 106(23), e187–e190.
Dall, C., Khan, M., Chen, C. A., & Angelos, M. G. (2016). Oxygen cycling to improve survival of stem cells for myocardial repair: A review. Life sciences, 153, 124–131.
Hashemi, S. S., Nazempour, M., Mehrabani, D., & Aghdam, R. M. (2019). The effect of allogenic human Wharton's jelly stem cells seeded onto acellular dermal matrix in healing of rat burn wounds. Journal of Cosmetic Dermatlogy, 13(1), 1–7.
White, S. J., & Chong, J. J. (2020). Growth factor therapy for cardiac repair: An overview of recent advances and future directions. Biophysical Reviews, 12, 1–11.
He, J. Q., Vu, D. M., Hunt, G., Chugh, A., Bhatnagar, A., & Bolli, R. (2011). Human cardiac stem cells isolated from atrial appendages stably express c-kit. PloS One, 6(11), e27719.
Ramalingam, M., Ramakrishna, S., & Best, S. (Eds.). (2012). Biomaterials and stem cells in regenerative medicine. CRC Press.
Mathiasen, A. B., Qayyum, A. A., Jørgensen, E., Helqvist, S., Fischer-Nielsen, A., Kofoed, K. F., Haack-Sørensen, M., Ekblond, A., & Kastrup, J. (2015). Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: A randomized placebo-controlled trial (MSC-HF trial). European Heart Journal, 36(27), 1744–1753.
Rodrigo, S. F., van Ramshorst, J., Hoogslag, G. E., Boden, H., Velders, M. A., Cannegieter, S. C., Roelofs, H., Al Younis, I., Dibbets-Schneider, P., Fibbe, W. E., & Zwaginga, J. J. (2013). Intramyocardial injection of autologous bone marrow-derived ex vivo expanded mesenchymal stem cells in acute myocardial infarction patients is feasible and safe up to 5 years of follow-up. Journal of Cardiovascular Translational Research, 6(5), 816–825.
Mukherjee, S., Venugopal, J. R., Ravichandran, R., Ramalingam, M., Raghunath, M., & Ramakrishna, S. (2013). Nanofiber technology for controlling stem cell functions and tissue engineering. Micro and nanotechnologies in engineering stem cells and tissues (pp. 27–51). Wiley.
Cheng, K., Malliaras, K., Smith, R. R., Shen, D., Sun, B., Blusztajn, A., Xie, Y., Ibrahim, A., Aminzadeh, M. A., Liu, W., & Li, T. S. (2014). Human cardiosphere-derived cells from advanced heart failure patients exhibit augmented functional potency in myocardial repair. JACC: Heart Failure, 2(1), 49–61.
Davis, D. R., Zhang, Y., Smith, R. R., Cheng, K., Terrovitis, J., Malliaras, K., Li, T. S., White, A., Makkar, R., & Marbán, E. (2009). Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PloS One, 4(9), e7195.
Funakoshi, S., Miki, K., Takaki, T., Okubo, C., Hatani, T., Chonabayashi, K., Nishikawa, M., Takei, I., Oishi, A., Narita, M., & Hoshijima, M. (2016). Enhanced engraftment, proliferation and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Scientific Reports, 6(1), 1–14.
Ja, K. M. M., Miao, Q., Tee, N. G. Z., Lim, S. Y., Nandihalli, M., Ramachandra, C. J., Mehta, A., & Shim, W. (2016). iPSC-derived human cardiac progenitor cells improve ventricular remodelling via angiogenesis and interstitial networking of infarcted myocardium. Journal of Cellular and Molecular Medicine, 20(2), 323–332.
Tang, J., Cui, X., Caranasos, T. G., Hensley, M. T., Vandergriff, A. C., Hartanto, Y., Shen, D., Zhang, H., Zhang, J., & Cheng, K. (2017). Heart repair using nanogel-encapsulated human cardiac stem cells in mice and pigs with myocardial infarction. ACS Nano, 11(10), 9738–9749.
Hortensius, R. A., Lin, W. H., & Ogle, B. M. (2019). Cardiac tissue engineering: A pathway for repair. Engineering in medicine (pp. 3–33). Academic Press.
Wu, K. H., Mo, X. M., Han, Z. C., & Zhou, B. (2011). Stem cell engraftment and survival in the ischemic heart. The Annals of Thoracic Surgery, 92(5), 1917–1925.
Zhang, H., Chen, H., Wang, W., Wei, Y., & Hu, S. (2010). Cell survival and redistribution after transplantation into damaged myocardium. Journal of Cellular and Molecular Medicine, 14(5), 1078–1082.
Shin, S. R., Jung, S. M., Zalabany, M., Kim, K., Zorlutuna, P., Kim, S. B., Nikkhah, M., Khabiry, M., Azize, M., Kong, J., & Wan, K. T. (2013). Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano, 7(3), 2369–2380.
Rana, D., Arulkumar, S., Vishwakarma, A., & Ramalingam, M. (2015). Considerations on designing scaffold for tissue engineering. Stem cell biology and tissue engineering in dental sciences (pp. 133–148). Academic Press.
Gaetani, R., Feyen, D. A., Verhage, V., Slaats, R., Messina, E., Christman, K. L., Giacomello, A., Doevendans, P. A., & Sluijter, J. P. (2015). Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials, 61, 339–348.
Zhang, D., Shadrin, I. Y., Lam, J., Xian, H. Q., Snodgrass, H. R., & Bursac, N. (2013). Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials, 34(23), 5813–5820.
Miyagi, Y., Chiu, L. L., Cimini, M., Weisel, R. D., Radisic, M., & Li, R. K. (2011). Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials, 32(5), 1280–1290.
Ahadian, S., Sadeghian, R. B., Salehi, S., Ostrovidov, S., Bae, H., Ramalingam, M., & Khademhosseini, A. (2015). Bioconjugated hydrogels for tissue engineering and regenerative medicine. Bioconjugate Chemistry, 26(10), 1984–2001.
Khan, M., Kwiatkowski, P., Rivera, B. K., & Kuppusamy, P. (2010). Oxygen and oxygenation in stem-cell therapy for myocardial infarction. Life Sciences, 87(9–10), 269–274.
Gholipourmalekabadi, M., Zhao, S., Harrison, B. S., Mozafari, M., & Seifalian, A. M. (2016). Oxygen-generating biomaterials: A new, viable paradigm for tissue engineering? Trends in Biotechnology, 34(12), 1010–1021.
Kim, H. Y., Kim, S. Y., Lee, H. Y., Lee, J. H., Rho, G. J., Lee, H. J., Lee, H. C., Byun, J. H., & Oh, S. H. (2019). Oxygen-releasing microparticles for cell survival and differentiation ability under hypoxia for effective bone regeneration. Biomacromolecules, 20(2), 1087–1097.
Kutala, V. K., Khan, M., Angelos, M. G., & Kuppusamy, P. (2007). Role of oxygen in postischemic myocardial injury. Antioxidants & Redox Signaling, 9(8), 1193–1206.
Beasley, R., Weatherall, M., Aldington, S., Mchaffie, D., & Robinson, G. (2007). Oxygen therapy in myocardial infarction: An historical perspective. Journal of the Royal Society of Medicine, 100(3), 130–133.
Maroko, P. R., Radvany, P. A. U. L. O., Braunwald, E. U. G. E. N. E., & Hale, S. L. (1975). Reduction of infarct size by oxygen inhalation following acute coronary occlusion. Circulation, 52(3), 360–368.
Alemdar, N., Leijten, J., Camci-Unal, G., Hjortnaes, J., Ribas, J., Paul, A., Mostafalu, P., Gaharwar, A. K., Qiu, Y., Sonkusale, S., & Liao, R. (2017). Oxygen-generating photo-cross-linkable hydrogels support cardiac progenitor cell survival by reducing hypoxia-induced necrosis. ACS Biomaterials Science & Engineering, 3(9), 1964–1971.
Lim, G. B. (2017). Supplemental oxygen in myocardial infarction. Nature Reviews Cardiology, 14(11), 632–632.
Bateman, N. T., & Leach, R. M. (1998). Acute oxygen therapy. BMJ, 317(7161), 798–801.
Shuvy, M., Atar, D., Gabriel Steg, P., Halvorsen, S., Jolly, S., Yusuf, S., & Lotan, C. (2013). Oxygen therapy in acute coronary syndrome: Are the benefits worth the risk? European Heart Journal, 34(22), 1630–1635.
Kelly, R. F., Hursey, T. L., Parrillo, J. E., & Schaer, G. L. (1995). Effect of 100% oxygen administration on infarct size and left ventricular function in a canine model of myocardial infarction and reperfusion. American Heart Journal, 130(5), 957–965.
Rawles, J. M., & Kenmure, A. C. (1976). Controlled trial of oxygen in uncomplicated myocardial infarction. British Medical Journal, 1(6018), 1121–1123.
Madias, J. E., & Hood, W. B., Jr. (1976). Reduction of precordial ST-segment elevation in patients with anterior myocardial infarction by oxygen breathing. Circulation, 53(3 Suppl), I198–I200.
Kenmure, A. C. F., Murdoch, W. R., Beattie, A. D., Marshall, J. C. B., & Cameron, A. J. V. (1968). Circulatory and metabolic effects of oxygen in myocardial infarction. British Medical Journal, 4(5627), 360–364.
Nicholson, C. (2004). A systematic review of the effectiveness of oxygen in reducing acute myocardial ischaemia. Journal of Clinical Nursing, 13(8), 996–1007.
Wijesinghe, M., Perrin, K., Ranchord, A., Simmonds, M., Weatherall, M., & Beasley, R. (2009). Routine use of oxygen in the treatment of myocardial infarction: Systematic review. Heart, 95(3), 198–202.
Cabello, J. B., Burls, A., Emparanza, J. I., Bayliss, S. E., & Quinn, T. (2016). Oxygen therapy for acute myocardial infarction. Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.CD007160.pub4
Li, W. F., Huang, Y. Q., & Feng, Y. Q. (2018). Oxygen therapy for patients with acute myocardial infarction: A meta-analysis of randomized controlled clinical trials. Coronary Artery Disease, 29(8), 652–656.
Grim, P. S., Gottlieb, L. J., Boddie, A., & Batson, E. (1990). Hyperbaric oxygen therapy. JAMA, 263(16), 2216–2220.
Stavitsky, Y., Shandling, A. H., Ellestad, M. H., Hart, G. B., Van Natta, B., Messenger, J. C., Strauss, M., Dekleva, M. N., Alexander, J. M., Mattice, M., & Clarke, D. (1998). Hyperbaric oxygen and thrombolysis in myocardial infarction: The ‘HOT MI’randomized multicenter study. Cardiology, 90(2), 131–136.
Khan, M., Meduru, S., Gogna, R., Madan, E., Citro, L., Kuppusamy, M. L., Sayyid, M., Mostafa, M., Hamlin, R. L., & Kuppusamy, P. (2012). Oxygen cycling in conjunction with stem cell transplantation induces NOS3 expression leading to attenuation of fibrosis and improved cardiac function. Cardiovascular Research, 93(1), 89–99.
Chen, C., Chen, W., Nong, Z., Ma, Y., Qiu, S., & Wu, G. (2016). Cardioprotective effects of combined therapy with hyperbaric oxygen and diltiazem pretreatment on myocardial ischemia-reperfusion injury in rats. Cellular Physiology and Biochemistry, 38(5), 2015–2029.
Dekleva, M., Neskovic, A., Vlahovic, A., Putnikovic, B., Beleslin, B., & Ostojic, M. (2004). Adjunctive effect of hyperbaric oxygen treatment after thrombolysis on left ventricular function in patients with acute myocardial infarction. American Heart Journal, 148(4), 589.
Vlahović, A., Nešković, A. N., Dekleva, M., Putniković, B., Popović, Z. B., Otašević, P., & Ostojić, M. (2004). Hyperbaric oxygen treatment does not affect left ventricular chamber stiffness after myocardial infarction treated with thrombolysis. American Heart Journal, 148(1), 85.
Bennett, M. H., Lehm, J. P., & Jepson, N. (2015). Hyperbaric oxygen therapy for acute coronary syndrome. Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.CD004818.pub4
Raut, M. S., & Maheshwari, A. (2016). Oxygen supplementation in acute myocardial infarction: To be or not to be? Annals of Cardiac Anaesthesia, 19(2), 342.
Abuzaid, A., Fabrizio, C., Felpel, K., Al Ashry, H. S., Ranjan, P., Elbadawi, A., Mohamed, A. H., Barssoum, K., & Elgendy, I. Y. (2018). Oxygen therapy in patients with acute myocardial infarction: A systemic review and meta-analysis. The American Journal of Medicine, 131(6), 693–701.
McNulty, P. H., King, N., Scott, S., Hartman, G., McCann, J., Kozak, M., Chambers, C. E., Demers, L. M., & Sinoway, L. I. (2005). Effects of supplemental oxygen administration on coronary blood flow in patients undergoing cardiac catheterization. American Journal of Physiology-Heart and Circulatory Physiology, 288(3), H1057–H1062.
McNulty, P. H., Robertson, B. J., Tulli, M. A., Hess, J., Harach, L. A., Scott, S., & Sinoway, L. I. (2007). Effect of hyperoxia and vitamin C on coronary blood flow in patients with ischemic heart disease. Journal of Applied Physiology, 102(5), 2040–2045.
Valko, M., Leibfritz, D., Moncol, J., Cronin, M. T., Mazur, M., & Telser, J. (2007). Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology, 39(1), 44–84.
Zweier, J. L., & Talukder, M. H. (2006). The role of oxidants and free radicals in reperfusion injury. Cardiovascular Research, 70(2), 181–190.
Atashi, F., Modarressi, A., & Pepper, M. S. (2015). The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells and Development, 24(10), 1150–1163.
Rodwell, V. W., & Kennelly, P. J. (2003). Enzymes: Mechanism of action. In R. K. Murray, D. K. Granner, P. A. Mayes, & V. W. Rodwell (Eds.), Harper’s illustrated biochemistry (26th ed., p. 57). Lange Medical Books/McGraw-Hill.
Gupta, A. S. (2019). Hemoglobin-based oxygen carriers: Current state-of-the-art and novel molecules. Shock (Augusta, Ga.), 52(1), 70.
Farris, A. L., Rindone, A. N., & Grayson, W. L. (2016). Oxygen delivering biomaterials for tissue engineering. Journal of Materials Chemistry B, 4(20), 3422–3432.
Hamilton, R. G., Kelly, N., Gawryl, M. S., & Rentko, V. T. (2001). Absence of immunopathology associated with repeated IV administration of bovine Hb-based oxygen carrier in dogs. Transfusion, 41(2), 219–225.
George, I., Yi, G. H., Schulman, A. R., Morrow, B. T., Cheng, Y., Gu, A., Zhang, G., Oz, M. C., Burkhoff, D., & Wang, J. (2006). A polymerized bovine hemoglobin oxygen carrier preserves regional myocardial function and reduces infarct size after acute myocardial ischemia. American Journal of Physiology-Heart and Circulatory Physiology, 291(3), H1126–H1137.
Li, T., Liu, J., & Yang, C. (2010). Pretreatment with hemoglobin-based oxygen carriers protect isolated rat heart from myocardial infarction. Artificial Cells, Blood Substitutes, and Biotechnology, 38(3), 115–118.
Caswell, J. E., Strange, M. B., Rimmer, D. M., III., Gibson, M. F., Cole, P., & Lefer, D. J. (2005). A novel hemoglobin-based blood substitute protects against myocardial reperfusion injury. American Journal of Physiology-Heart and Circulatory Physiology, 288(4), H1796–H1801.
Njoku, M., Peter, D. S., & Mackenzie, C. F. (2015). Haemoglobin-based oxygen carriers: Indications and future applications. British Journal of Hospital Medicine, 76(2), 78–83.
Cabrales, P., & Intaglietta, M. (2013). Blood substitutes: Evolution from non-carrying to oxygen and gas carrying fluids. ASAIO Journal (American Society for Artificial Internal Organs: 1992), 59(4), 337–354.
Alayash, A. I. (2014). Blood substitutes: Why haven’t we been more successful? Trends in Biotechnology, 32(4), 177–185.
García-Ruiz, J. M., Galán-Arriola, C., Fernández-Jiménez, R., Aguero, J., Sánchez-González, J., García-Alvarez, A., Nuno-Ayala, M., Dubé, G. P., Zafirelis, Z., López-Martín, G. J., & Bernal, J. A. (2017). Bloodless reperfusion with the oxygen carrier HBOC-201 in acute myocardial infarction: A novel platform for cardioprotective probes delivery. Basic Research in Cardiology, 112(2), 17.
te Lintel Hekkert, M., Dubé, G. P., Regar, E., de Boer, M., Vranckx, P., van der Giessen, W. J., Serruys, P. W., & Duncker, D. J. (2010). Preoxygenated hemoglobin-based oxygen carrier HBOC-201 annihilates myocardial ischemia during brief coronary artery occlusion in pigs. American Journal of Physiology-Heart and Circulatory Physiology, 298, H1103–H1113.
Mackenzie, C. F., Dubé, G. P., Pitman, A., & Zafirelis, M. (2019). Users guide to pitfalls and lessons learned about HBOC-201 during clinical trials, expanded access, and clinical use in 1,701 patients. Shock, 52(1S), 92–99.
Li, T., Yu, R., Zhang, H. H., Wang, H., Liang, W. G., Yang, X. M., & Yang, C. M. (2006). A method for purification and viral inactivation of human placenta hemoglobin. Artificial Cells, Blood Substitutes, and Biotechnology, 34(2), 175–188.
Yang, Q., Wu, W., Li, Q., Chen, C., Zhou, R., Qiu, Y., Luo, M., Tan, Z., Li, S., Chen, G., & Zhou, W. (2015). High-dose polymerized hemoglobin fails to alleviate cardiac ischemia/reperfusion injury due to induction of oxidative damage in coronary artery. Oxidative Medicine and Cellular Longevity, 2015, 125106.
Tsuchida, E., Sou, K., Nakagawa, A., Sakai, H., Komatsu, T., & Kobayashi, K. (2009). Artificial oxygen carriers, hemoglobin vesicles and albumin− hemes, based on bioconjugate chemistry. Bioconjugate Chemistry, 20(8), 1419–1440.
Fan, Z., Xu, Z., Niu, H., Gao, N., Guan, Y., Li, C., Dang, Y., Cui, X., Liu, X. L., Duan, Y., & Li, H. (2018). An injectable oxygen release system to augment cell survival and promote cardiac repair following myocardial infarction. Scientific Reports, 8(1), 1–22.
Estep, T. N. (2019). Haemoglobin-based oxygen carriers and myocardial infarction. Artificial cells, nanomedicine, and biotechnology, 47(1), 593–601.
Momen, A., Mascarenhas, V., Gahremanpour, A., Gao, Z., Moradkhan, R., Kunselman, A., Boehmer, J. P., Sinoway, L. I., & Leuenberger, U. A. (2009). Coronary blood flow responses to physiological stress in humans. American Journal of Physiology-Heart and Circulatory Physiology, 296(3), H854–H861.
Barsotti, M. C., Felice, F., Balbarini, A., & Di Stefano, R. (2011). Fibrin as a scaffold for cardiac tissue engineering. Biotechnology and Applied Biochemistry, 58(5), 301–310.
Karam, J. P., Muscari, C., Sindji, L., Bastiat, G., Bonafè, F., Venier-Julienne, M. C., & Montero-Menei, N. C. (2014). Pharmacologically active microcarriers associated with thermosensitive hydrogel as a growth factor releasing biomimetic 3D scaffold for cardiac tissue-engineering. Journal of Controlled Release, 192, 82–94.
Jiang, L., Chen, D., Wang, Z., Zhang, Z., Xia, Y., Xue, H., & Liu, Y. (2019). Preparation of an electrically conductive graphene oxide/chitosan scaffold for cardiac tissue engineering. Applied Biochemistry and Biotechnology, 188(4), 952–964.
Seif-Naraghi, S. B., Salvatore, M. A., Schup-Magoffin, P. J., Hu, D. P., & Christman, K. L. (2010). Design and characterization of an injectable pericardial matrix gel: A potentially autologous scaffold for cardiac tissue engineering. Tissue Engineering Part A, 16(6), 2017–2027.
Lovett, M., Lee, K., Edwards, A., & Kaplan, D. L. (2009). Vascularization strategies for tissue engineering. Tissue Engineering Part B: Reviews, 15(3), 353–370.
Ward, C. L., Corona, B. T., Yoo, J. J., Harrison, B. S., & Christ, G. J. (2013). Oxygen generating biomaterials preserve skeletal muscle homeostasis under hypoxic and ischemic conditions. PloS One, 8(8), e72485.
Lee, H. Y., Kim, H. W., Lee, J. H., & Oh, S. H. (2015). Controlling oxygen release from hollow microparticles for prolonged cell survival under hypoxic environment. Biomaterials, 53, 583–591.
Shiekh, P. A., Singh, A., & Kumar, A. (2018). Oxygen-releasing antioxidant cryogel scaffolds with sustained oxygen delivery for tissue engineering applications. ACS Applied Materials & Interfaces, 10(22), 18458–18469.
Abdi, S. I. H., Ng, S. M., & Lim, J. O. (2011). An enzyme-modulated oxygen-producing micro-system for regenerative therapeutics. International Journal of Pharmaceutics, 409(1–2), 203–205.
Li, Z., Guo, X., & Guan, J. (2012). An oxygen release system to augment cardiac progenitor cell survival and differentiation under hypoxic condition. Biomaterials, 33(25), 5914–5923.
Abdi, S. I. H., Choi, J. Y., Lau, H. C., & Lim, J. O. (2013). Controlled release of oxygen from PLGA-alginate layered matrix and its in vitro characterization on the viability of muscle cells under hypoxic environment. Tissue Engineering and Regenerative Medicine, 10(3), 131–138.
Oh, S. H., Ward, C. L., Atala, A., Yoo, J. J., & Harrison, B. S. (2009). Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials, 30(5), 757–762.
Pedraza, E., Coronel, M. M., Fraker, C. A., Ricordi, C., & Stabler, C. L. (2012). Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proceedings of the National Academy of Sciences USA, 109(11), 4245–4250.
Lee, Y., Son, J. Y., Kang, J. I., Park, K. M., & Park, K. D. (2018). Hydrogen peroxide–releasing hydrogels for enhanced endothelial cell activities and neovascularization. ACS Applied Materials & Interfaces, 10(21), 18372–18379.
Liu, G., & Porterfield, D. M. (2014). Oxygen enrichment with magnesium peroxide for minimizing hypoxic stress of flooded corn. Journal of Plant Nutrition and Soil Science, 177(5), 733–740.
Harrison, B. S., Eberli, D., Lee, S. J., Atala, A., & Yoo, J. J. (2007). Oxygen producing biomaterials for tissue regeneration. Biomaterials, 28(31), 4628–4634.
Ghelich, R., Aghdam, R. M., Torknik, F. S., & Jahannama, M. R. (2016). Low temperature carbothermal reduction synthesis of ZrC nanofibers via cyclized electrospun PVP/Zr(OPr)4 hybrid. International Journal of Applied Ceramic Technology, 13(2), 352–358.
Touri, M., Moztarzadeh, F., Osman, N. A. A., Dehghan, M. M., & Mozafari, M. (2019). Optimisation and biological activities of bioceramic robocast scaffolds provided with an oxygen-releasing coating for bone tissue engineering applications. Ceramics International, 45(1), 805–816.
Mohseni-Vadeghani, E., Karimi-Soflou, R., Khorshidi, S., & Karkhaneh, A. (2021). Fabrication of oxygen and calcium releasing microcarriers with different internal structures for bone tissue engineering: Solid filled versus hollow microparticles. Colloids and Surfaces B: Biointerfaces, 197, 111376.
Choi, J., Hong, G., Kwon, T., & Lim, J. O. (2018). Fabrication of oxygen releasing scaffold by embedding H2O2-PLGA microspheres into alginate-based hydrogel sponge and its application for wound healing. Applied Sciences, 8(9), 1492.
Kang, J. I., Park, K. M., & Park, K. D. (2019). Oxygen-generating alginate hydrogels as a bioactive acellular matrix for facilitating wound healing. Journal of Industrial and Engineering Chemistry, 69, 397–404.
McQuilling, J. P., Sittadjody, S., Pendergraft, S., Farney, A. C., & Opara, E. C. (2017). Applications of particulate oxygen-generating substances (POGS) in the bioartificial pancreas. Biomaterials Science, 5(12), 2437–2447.
Seifu, D. G., Isimjan, T. T., & Mequanint, K. (2011). Tissue engineering scaffolds containing embedded fluorinated-zeolite oxygen vectors. Acta Biomaterialia, 7(10), 3670–3678.
Fu, H., Fu, J., Ma, S., Wang, H., Lv, S., & Hao, Y. (2020). An ultrasound activated oxygen generation nanosystem specifically alleviates myocardial hypoxemia and promotes cell survival following acute myocardial infarction. Journal of Materials Chemistry B, 8(28), 6059–6068.
Montazeri, L., Kowsari-Esfahan, R., Pahlavan, S., Sobat, M., Rabbani, S., Ansari, H., Varzideh, F., Barekat, M., Rajabi, S., Navaee, F., & Bonakdar, S. (2021). Oxygen-rich environment ameliorates cell therapy outcomes of cardiac progenitor cells for myocardial infarction. Materials Science and Engineering: C, 121, 111836.
Ding, J., Yao, Y., Li, J., Duan, Y., Nakkala, J. R., Feng, X., Cao, W., Wang, Y., Hong, L., Shen, L., & Mao, Z. (2020). A reactive oxygen species scavenging and O2 generating injectable hydrogel for myocardial infarction treatment in vivo. Small (Weinheim an der Bergstrasse, Germany), 16(48), 2005038.
Erdem, A., Darabi, M. A., Nasiri, R., Sangabathuni, S., Ertas, Y. N., Alem, H., Hosseini, V., Shamloo, A., Nasr, A. S., Ahadian, S., & Dokmeci, M. R. (2020). 3D bioprinting of oxygenated cell-laden gelatin methacryloyl constructs. Advanced Healthcare Materials, 9(15), 1901794.
Camci-Unal, G., Alemdar, N., Annabi, N., & Khademhosseini, A. (2013). Oxygen-releasing biomaterials for tissue engineering. Polymer International, 62(6), 843–848.
Willemen, N. G., Hassan, S., Gurian, M., Li, J., Allijn, I. E., Shin, S. R., & Leijten, J. (2021). Oxygen-releasing biomaterials: Current challenges and future applications. Trends in Biotechnology. https://doi.org/10.1016/j.tibtech.2021.01.007
Suvarnapathaki, S., Wu, X., Lantigua, D., Nguyen, M. A., & Camci-Unal, G. (2019). Breathing life into engineered tissues using oxygen-releasing biomaterials. NPG Asia Materials, 11(1), 1–18.
Ashammakhi, N., Darabi, M. A., Kehr, N. S., Erdem, A., Hu, S. K., Dokmeci, M. R., Nasr, A. S., & Khademhosseini, A. (2019). Advances in controlled oxygen generating biomaterials for tissue engineering and regenerative therapy. Biomacromolecules, 21(1), 56–72.
Razavi, M., Primavera, R., Kevadiya, B. D., Wang, J., Buchwald, P., & Thakor, A. S. (2020). A collagen based cryogel bioscaffold that generates oxygen for islet transplantation. Advanced Functional Materials, 30(15), 1902463.
Zhang, M., Kiratiwongwan, T., & Shen, W. (2020). Oxygen-releasing polycaprolactone/calcium peroxide composite microspheres. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 108(3), 1097–1106.
Acknowledgements
The authors would like to thank the National Institute for Medical Research Development (NIMAD) (Grant No. 972838) for the financial support of this research project.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Handling Editor: Vincent FM Segers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jenabi, A., Mehdinavaz Aghdam, R., Ebrahimi, S.A.S. et al. Oxygen Delivery Approaches to Augment Cell Survival After Myocardial Infarction: Progress and Challenges. Cardiovasc Toxicol 22, 207–224 (2022). https://doi.org/10.1007/s12012-021-09696-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-021-09696-5