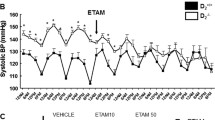
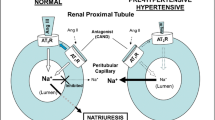
Abstract
The role of dopamine receptors in blood pressure regulation is well established. Genetic ablation of both dopamine D1-like receptor subtypes (D1, D5) and D2-like receptor subtypes (D2, D3, D4) results in a hypertensive phenotype in mice. This review focuses on the dopamine D1-like receptor subtypes D1 and D5 (especially D1 receptors), as they play a major role in regulating sodium homeostasis and blood pressure. Studies mostly describing the role of renal dopamine D1-like receptors are included, as the kidneys play a pivotal role in the maintenance of sodium homeostasis and the long-term regulation of blood pressure. We also attempt to describe the interaction between D1-like receptors and other proteins, especially angiotensin II type 1 and type 2 receptors, which are involved in the maintenance of sodium homeostasis and blood pressure. Finally, we discuss a new concept of renal D1 receptor regulation in hypertension that involves oxidative stress mechanisms.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gong M, Hubner N. Molecular genetics of human hypertension. Clin Sci. 2006;110:315–26.
Zeng C, Eisner GM, Felder RA, Jose PA. D3 dopamine receptor and essential hypertension. Curr Hypertens Rev. 2006;2:247–53.
Zeng C, Felder RA, Jose PA. A new approach for treatment of hypertension: modifying D1 dopamine receptor function. Cardiovasc Hematol Agents Med Chem. 2006;4:369–77.
Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med. 2003;228:134–42.
Weinberger MH, Fineberg NS, Fineberg SE, et al. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–32.
de la Sierra A, Giner V, Bragulat E, et al. Lack of correlation between two methods for the assessment of salt sensitivity in essential hypertension. J Hum Hypertens. 2002;16:255–60.
Felder CC, Jose PA, Axelrod J. The dopamine-1 agonist, SKF 82526, stimulates phospholipase-C activity independent of adenylate cyclase. J Pharmacol Exp Ther. 1989;248:171–5.
Bertorello A, Aperia A. Na+−K+−ATPase is an effector protein for protein kinase C in renal proximal tubule cells. Am J Physiol. 1989;256:F370–3.
Hussain T, Lokhandwala MF. Renal dopamine DA1 receptor coupling with GS and Gq/11 proteins in spontaneously hypertensive rats. Am J Physiol. 1997;272:F339–46.
Lezcano N, Mrzljak L, Eubanks S, Levenson R, Goldman-Rakic P, Bergson C. Dual signaling regulated by calcyon, a D1 dopamine receptor interacting protein. Science. 2000;287:1660–4.
Zeng C, Zhang M, Asico LD, et al. The dopaminergic system in hypertension. Clin Sci (Lond). 2007;112:583–97.
O’Connell DP, Botkin SJ, Ramos SI, et al. Localization of dopamine D1A receptor protein in rat kidneys. Am J Physiol. 1995;268:F1185–97.
Ozono R, O’Connell DP, Wang ZQ, et al. Localization of the dopamine D1 receptor protein in the human heart and kidney. Hypertension. 1997;30:725–9.
Yamaguchi I, Jose PA, Mouradian MM, et al. Expression of dopamine D1A receptor gene in proximal tubule of rat kidneys. Am J Physiol. 1993;264:F280–5.
Ricci A, Amenta F, Bronzetti E, et al. Age-related changes of dopamine receptor protein immunoreactivity in the rat mesenteric vascular tree. Mech Ageing Dev. 2002;123:537–46.
Amenta F, Barili P, Bronzetti E, et al. Localization of dopamine receptor subtypes in systemic arteries. Clin Exp Hypertens. 2000;22:277–88.
Kim MO, Koh PO, Kim JH, et al. Localization of dopamine D1 and D2 receptor mRNAs in the rat systemic and pulmonary vasculatures. Mol Cell. 1999;9:417–21.
Zheng S, Yu P, Zeng C, et al. Gα12- and Gα13-protein subunit linkage of D5 dopamine receptors in the nephron. Hypertension. 2003;41:604–10.
Amenta F. Light microscope autoradiography of peripheral dopamine receptor subtypes. Clin Exp Hypertens. 1997;19:27–41.
Ricci A, Chiandussi L, Schena M, et al. Dopamine D5 receptor expression is unchanged in peripheral blood lymphocytes in essential hypertension. Clin Exp Hypertens. 1995;17:1157–72.
Ortiz PA, Garvin JL. Intrarenal transport and vasoactive substances in hypertension. Hypertension. 2001;38:621–4.
Aviv A, Hollenberg NK, Weder A. Urinary potassium excretion and sodium sensitivity in blacks. Hypertension. 2004;43:707–13.
Redon J, Lurbe E. Nocturnal blood pressure versus nondipping pattern: what do they mean? Hypertension. 2008;511:41–2.
Kuchel OG, Kuchel GA. Peripheral dopamine in pathophysiology of hypertension. Interaction with aging and lifestyle. Hypertension. 1991;18:709–21.
Damasceno A, Santos A, Serrao P, et al. Deficiency of renal dopaminergic-dependent natriuretic response to acute sodium load in black salt-sensitive subjects in contrast to salt-resistant subjects. J Hypertens. 1999;17:1995–2001.
Clark BA, Rosa RM, Epstein FH, et al. Altered dopaminergic responses in hypertension. Hypertension. 1992;19:589–94.
Romero-Vecchione E, Vasquez J, Lema G, et al. Low urinary dopamine excretion associated to low sodium excretion in normotensive Piaroa Amazonian ethnia compared to urban subjects. Invest Clin. 1995;36:61–71.
Gill Jr JR. Grossman E, Goldstein DS: High urinary dopa and low urinary dopamine-to-dopa ratio in salt-sensitive hypertension. Hypertension. 1991;18:614–21.
Saito I, Takeshita E, Saruta T, et al. Urinary dopamine excretion in normotensive subjects with or without family history of hypertension. J Hypertens. 1986;4:57–60.
Saito I, Itsuji S, Takeshita E, et al. Increased urinary dopamine excretion in young patients with essential hypertension. Clin Exp Hypertens. 1994;16:29–39.
Grossman E, Hoffman A, Tamrat M, et al. Endogenous dopa and dopamine responses to dietary salt loading in salt-sensitive rats. J Hypertens. 1991;9:259–63.
Stier Jr CT. Itskovitz HD, Chen YH: Urinary dopamine and sodium excretion in spontaneously hypertensive rats. Clin Exp Hypertens. 1993;15:105–23.
Yoshimura M, Kambara S, Okabayashi H, et al. Effect of decreased dopamine synthesis on the development of hypertension induced by salt loading in spontaneously hypertensive rats. Clin Exp Hypertens. 1987;A9:1141–57.
Jose PA, Eisner GM, Drago J, et al. Dopamine receptor signaling defects in spontaneous hypertension. Am J Hypertens. 1996;9:400–5.
Lifton RP, Wilson FH, Choate KA, Geller DS. Salt and blood pressure: new insight from human genetic studies. Cold Spring Harbor Symp Quant Biol. 2002;67:445–50.
Siragy HM, Felder RA, Howell NL, et al. Evidence that intrarenal dopamine acts as a paracrine substance at the renal tubule. Am J Physiol. 1989;257:F469–77.
Jose PA, Asico LD, Eisner GM, et al. Effects of costimulation of dopamine D1- and D2-like receptors on renal function. Am J Physiol. 1998;275:R986–9.
Hansell P, Fasching A. The effect of dopamine receptor blockade on natriuresis is dependent on the degree of hypervolemia. Kidney Int. 1991;39:253–8.
Sunahara RK, Guan HC, O’Dowd BF, et al. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991;350:614–9.
Sanada H, Xu J, Watanabe H, et al. Differential expression and regulation of dopamine-1 (D-1) and dopamine-5 (D-5) receptor function in human kidney. Am J Hypertens. 2000;13:156A.
Tiberi M, Caron MG. High agonist-independent activity is a distinguishing feature of the dopamine D1B receptor subtype. J Biol Chem. 1994;269:27925–31.
Moore JH, Williams SM. New strategies for identifying gene–gene interactions in hypertension. Ann Med. 2002;34:88–99.
Ladines CA, Zeng C, Asico LD, et al. Impaired renal D1-like and D2-like dopamine receptor interaction in the spontaneously hypertensive rat. Am J Physiol Regulatory Integrative Comp Physiol. 2001;281:R1071–8.
Eklof AC. The natriuretic response to a dopamine DA1 agonist requires endogenous activation of dopamine DA2 receptors. Acta Physiol Scand. 1997;160:311–4.
Bacic D, Kaissling B, McLeroy P, et al. Dopamine acutely decreases apical membrane Na/H exchanger NHE3 protein in mouse renal proximal tubule. Kidney Int. 2003;64:2133–41.
Felder CC, Albrecht FE, Campbell T, et al. Cyclic AMP-independent, G protein-linked inhibition of Na+/H+ exchange in renal brush border by D1 dopamine agonists. Am J Physiol. 1993;264:F1032–7.
Bacic D, Capuano P, Baum M, et al. Activation of dopamine D1-like receptors induces acute internalization of the renal Na+/phosphate cotransporter NaPi-IIa in mouse kidney and OK cells. Am J Physiol Renal Physiol. 2005;288:F740–7.
Pedrosa R, Jose PA, Soares-da-Silva P. Defective D1-like receptor-mediated inhibition of the Cl−/HCO3− exchanger in immortalized SHR proximal tubular epithelial cells. Am J Physiol Renal Physiol. 2004;286:F1120–6.
Efendiev R, Cinelli AR, Leibiger IB, et al. FRET analysis reveals a critical conformational change within the Na, K-ATPase α1 subunit N-terminus during GPCR-dependent endocytosis. FEBS Lett. 2006;580:5067–70.
Asghar M, Hussain T, Lokhandwala MF. Activation of dopamine D1-like receptor causes phosphorylation of α1-subunit of Na+, K+−ATPase in rat renal proximal tubules. Eur J Pharmacol. 2001;411:61–6.
Kunimi M, Seki G, Hara C, et al. Dopamine inhibits renal Na+:HCO3− cotransporter in rabbits and normotensive rats but not in spontaneously hypertensive rats. Kidney Int. 2000;57:534–43.
Wang ZQ, Felder RA, Carey RM. Selective inhibition of the renal dopamine subtype D1A receptor induces antinatriuresis in conscious rats. Hypertension. 1999;33:504–10.
Crambert S, Sjöberg A, Eklöf AC, et al. Prolactin and dopamine 1-like receptor interaction in renal proximal tubular cells. Am J Physiol Renal Physiol. 2010;299:F49–54.
•• Li H, Armando I, Yu P, et al. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J Clin Invest. 2008;118:2180–9. This article demonstrates counterregulation between the dopamine D5 receptor and the AT1 receptor as a mechanism of blood pressure regulation.
Zeng C, Wang Z, Hopfer U, et al. Rat strain effects of AT1 receptor activation on D1 dopamine receptors in immortalized renal proximal tubule cells. Hypertension. 2005;46:799–805.
Zeng C, Liu Y, Wang Z, et al. Activation of D3 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Circ Res. 2006;99:494–500.
• Khan F, Spicarova Z, Zelenin S, et al. Negative reciprocity between angiotensin II type 1 and dopamine D1 receptors in rat renal proximal tubule cells. Am J Physiol Renal Physiol. 2008;295:F1110–6. This study shows that the dopamine D1 receptor and the AT1 receptor coimmunoprecipitate, and that D1-like stimulation counterregulates a well-known effect of the AT1 receptor, namely calcium signaling.
Hollon TR, Bek MJ, Lachowicz JE, Ariano MA, Mezey E, Ramachandran R, et al. Westphal H, Jose PA, Sibley DR: Mice lacking D5 dopamine receptors have increased sympathetic tone and are hypertensive. J Neurosci. 2002;22:10801–10.
Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Escano CS, et al. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R96–R104.
Zeng C, Yang Z, Wang Z, Jones J, Wang X, Altea J, et al. Interaction of angiotensin II type 1 and D5 dopamine receptors in renal proximal tubule cells. Hypertension. 2005;45:804–10.
• Wang X, Luo Y, Escano CS, Yang Z, Asico L, Li H, et al. Upregulation of renal sodium transporters in D5 dopamine receptor-deficient mice. Hypertension. 2010;55:1431–7. This article shows that ablation of the D5 receptor in mice increases both AT1 receptor and sodium transporters/channels contributing to hypertension.
• Chugh G, Lokhandwala MF, Asghar M. Oxidative stress alters renal D1 and AT1 receptor functions and increases blood pressure in old rats. Am J Physiol Renal Physiol. 2011;300:F133–138. This article investigates the causal role of age-associated oxidative stress in exaggerated renal AT1 receptor function and diminished D1 receptor function, contributing to hypertension in old rats.
• Hakam AC, Siddiqui AH, Hussain T: Renal angiotensin II AT2 receptors promote natriuresis in streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol 2006, 290:F503–F508. This article describes the mechanism of AT2 receptor-mediated natriuresis and diuresis.
Hakam AC, Hussain T. Renal angiotensin II type-2 receptors are upregulated and mediate the candesartan-induced natriuresis/diuresis in obese Zucker rats. Hypertension. 2005;45:270–5.
Salomone LJ, Howell NL, McGrath HE, et al. Intrarenal dopamine D1-like receptor stimulation induces natriuresis via an angiotensin type-2 receptor mechanism. Hypertension. 2007;49:155–61.
Padia SH, Howell NL, Siragy HM, Carey RM. Renal angiotensin type 2 receptors mediate natriuresis via angiotensin III in the angiotensin II type 1 receptor-blocked rat. Hypertension. 2006;47:537–44.
Citarella MR, Choi MR, Gironacci MM, et al. Urodilatin and dopamine: a new interaction in the kidney. Regul Pept. 2009;153:19–24.
Yao B, Harris RC, Zhang MZ. Intrarenal dopamine attenuates deoxycorticosterone acetate/high salt-induced blood pressure elevation in part through activation of a medullary cyclooxygenase 2 pathway. Hypertension. 2009;54:1077–83.
Kirchheimer C, Mendez CF, Acquier A, et al. Role of 20-HETE in D1/D2 dopamine receptor synergism resulting in the inhibition of Na+−K+−ATPase activity in the proximal tubule. Am J Physiol Renal Physiol. 2007;292:F1435–42.
Venkatakrishnan U, Chen C, Lokhandwala MF. The role of intrarenal nitric oxide in the natriuretic response to dopamine receptor activation. Clin Exp Hypertens. 2000;22:309–24.
Marwaha A, Lokhandwala MF. Diminished natriuretic response to dopamine D1 receptor agonist, SKF-38393 in obese Zucker rats. Clin Exp Hypertens. 2003;25:509–15.
Marwaha A, Banday AA, Lokhandwala MF. Reduced renal dopamine D1 receptor function in streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol. 2004;286:F451–7.
Moreira-Rodrigues M, Quelhas-Santos J, Serrão P, Fernandes-Cerqueira C, Sampaio-Maia B, Pestana M. Glycaemic control with insulin prevents the reduced renal dopamine D1 receptor expression and function in streptozotocin-induced diabetes. Nephrol Dial Transplant. 2010;25:2945–53.
Hahn RA, Wardell Jr JR. Sarau HM, Ridley PT: Characterization of the peripheral and central effects of SK&F 82526, a novel dopamine receptor agonist. J Pharmacol Exp Ther. 1982;223:305–13.
Murphy MB, Murray C, Shorten GD. Fenoldopam: a selective peripheral dopamine-receptor agonist for the treatment of severe hypertension. N Engl J Med. 2001;345:1548–57.
Yaqoob M, McClelland P, Ahmad R. Delayed recovery of renal function in patients with acute renal failure due to accelerated hypertension. Postgrad Med J. 1991;67:829–32.
Bakir AA, Bazilinski N, Dunea G. Transient and sustained recovery from renal shutdown in accelerated hypertension. Am J Med. 1986;80:172–6.
Reisin E, Huth MM, Nguyen BP, Weed SG, Gonzalez FM. Intravenous fenoldopam versus sodium nitroprusside in patients with severe hypertension. Hypertension. 1990;15(Suppl):I59–62.
Zeng C, Armando I, Luo Y, et al. Dysregulation of dopamine dependent mechanisms as a determinant of hypertension: studies in dopamine receptor knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H551–69.
Ueda A, Ozono R, Oshima T, et al. Disruption of the type 2 dopamine receptor gene causes a sodium-dependent increase in blood pressure in mice. Am J Hypertens. 2003;16:853–8.
Staudacher T, Pech B, Tappe M, et al. Arterial blood pressure and renal sodium excretion in dopamine D3 receptor knockout mice. Hypertens Res. 2007;30:93–101.
Sunahara RK, Niznik HB, Weiner DM, et al. Human dopamine D1 receptor encoded by an intronless gene on chromosome 5. Nature. 1990;347:80–3.
•• Kiryluk K: Renal function and genetic variation in dopamine D(1) receptor: is the case strong enough? Kidney Int 2009, 76:1019–1022. This article reviews the role of DRD1 gene polymorphisms in blood pressure regulation.
Fung MM, Rana BK, Tang C-M, et al. Dopamine D1 receptor (DRD1) genetic polymorphism: pleiotropic effects on heritable renal traits. Kidney Int. 2009;76:1070–80.
Rao F, Wessel J, Wen G, et al. Renal albumin excretion: twin studies identify influences of heredity, environment, and adrenergic pathway polymorphism. Hypertension. 2007;49:1015–31.
Staessen JA, Kuznetsova T, Zhang H, et al. Blood pressure and renal sodium handling in relation to genetic variation in the DRD1 promoter and GRK4. Hypertension. 2008;51:1643–50.
Sen S, Nesse R, Sheng L, et al. Association between a dopamine-4 receptor polymorphism and blood pressure. Am J Hypertens. 2005;18:1206–10.
Banday AA, Lau YS, Lokhandwala MF. Oxidative stress causes renal dopamine D1 receptor dysfunction and salt-sensitive hypertension in Sprague-Dawley rats. Hypertension. 2008;51:367–75.
Banday AA, Lokhandwala MF. Dopamine receptors and hypertension. Curr Hypertens Rep. 2008;10:268–75.
• Jose PA, Soares-da-Silva P, Eisner GM, Felder RA: Dopamine and G protein-coupled receptor kinase 4 in the kidney: role in blood pressure regulation. Biochim Biophys Acta 2010, 1802:1259–1267. This article reviews the role of GRK4 gene variants in regulating blood pressure.
O’Connell DP, Ragsdale NV, Boyd DG, et al. Differential human renal tubular responses to dopamine type 1 receptor stimulation are determined by blood pressure status. Hypertension. 1997;29(1 Pt 1):115–22.
Felder RA, Sanada H, Xu J, et al. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci USA. 2002;99:3872–7.
Felder RA, Seikaly MG, Cody P, et al. Attenuated renal response to dopaminergic drugs in spontaneously hypertensive rats. Hypertension. 1990;15:560–9.
Chen C, Lokhandwala MF. An impairment of renal tubular DA-1 receptor function as the causative factor for diminished natriuresis to volume expansion in spontaneously hypertensive rats. Clin Exp Hypertens. 1992;14:615–28.
Nishi A, Eklof AC, Bertorello AM, Aperia A. Dopamine regulation of renal Na+, K+−ATPase activity is lacking in Dahl salt-sensitive rats. Hypertension. 1993;21:767–71.
Chen C, Beach RE, Lokhandwala MF. Dopamine fails to inhibit renal tubular sodium pump in hypertensive rats. Hypertension. 1993;21:364–72.
Xu J, Li XX, Albrecht FE, et al. Dopamine1 receptor, GSα, and Na+−H+ exchanger interactions in the kidney in hypertension. Hypertension. 2000;36:395–9.
Sanada H, Jose PA, Hazen-Martin D, et al. Dopamine-1 receptor coupling defect in renal proximal tubule cells in hypertension. Hypertension. 1999;33:1036–42.
Zhu H, Lu Y, Wang X, et al. The G protein-coupled receptor kinase 4 gene modulates stress-induced sodium excretion in black normotensive adolescents. Pediatr Res. 2006;60:440–2.
Sanada H, Yatabe J, Midorikawa S, et al. Single-nucleotide polymorphisms for diagnosis of salt-sensitive hypertension. Clin Chem. 2006;52:352–60.
Rana BK, Insel PA, Payne SH, et al. Population-based sample reveals gene-gender interactions in blood pressure in white Americans. Hypertension. 2007;49:96–106.
Marques FZ, Campain AE, Yang YH, et al. Meta-analysis of genome-wide gene expression differences in onset and maintenance phases of genetic hypertension. Hypertension. 2010;56:319–24.
Bengra C, Mifflin TE, Khripin Y, et al. Genotyping of essential hypertension single-nucleotide polymorphisms by a homogeneous PCR method with universal energy transfer primers. Clin Chem. 2002;48:2131–40.
• Asghar M, Chugh G, Lokhandwala MF: Inflammation compromises renal dopamine D1 receptor function in rats. Am J Physiol Renal Physiol 2009, 297:F1543-F1549. This paper describes the role of inflammation and redox-sensitive transcription factors, NFκB and Nrf2, on D1 receptor function regulation.
White BH, Sidhu A. Increased oxidative stress in renal proximal tubules of the spontaneously hypertensive rat: a mechanism for defective dopamine D1A receptor/G-protein coupling. J Hypertens. 1998;16:1659–65.
Asghar M, Banday AA, Fardoun RZ, Lokhandwala MF. Hydrogen peroxide causes uncoupling of dopamine D1-like receptors from G proteins via a mechanism involving protein kinase C and G-protein-coupled receptor kinase 2. Free Radic Biol Med. 2006;40:13–20.
Fardoun RZ, Asghar M, Lokhandwala M. Role of oxidative stress in defective renal dopamine D1 receptor-G protein coupling and function in old Fischer 344 rats. Am J Physiol Renal Physiol. 2006;291:F945–51.
Banday AA, Marwaha A, Tallam LS, Lokhandwala MF. Tempol reduces oxidative stress, improves insulin sensitivity, decreases renal dopamine D1 receptor hyperphosphorylation, and restores D1 receptor-G-protein coupling and function in obese Zucker rats. Diabetes. 2005;54:2219–26.
George L, Lokhandwala MF, Asghar M. Exercise activates redox-sensitive transcription factors and restores renal D1 receptor function in old rats. Am J Physiol Renal Physiol. 2009;297:F1174–80.
Muhammad AB, Lokhandwala MF, Banday AA. Exercise reduces oxidative stress but does not alleviate hyperinsulinemia or renal dopamine D1 receptor dysfunction in obese rats. Am J Physiol Renal Physiol. 2011;300:F98–F104.
Watanabe H, Xu J, Bengra C, Jose PA, Felder RA. Desensitization of human renal D1 dopamine receptors by G protein-coupled receptor kinase 4. Kidney Int. 2002;62:790–8.
Trivedi M, Lokhandwala MF. Rosiglitazone restores renal D1A receptor-Gs protein coupling by reducing receptor hyperphosphorylation in obese rats. Am J Physiol Renal Physiol. 2005;289:F298–304.
Yasunari K, Kohno M, Kano H, Minami M, Yoshikawa J. Dopamine as a novel antioxidative agent for rat vascular smooth muscle cells through dopamine D(1)-like receptors. Circulation. 2000;101:2302–8.
Yatabe J, Sanada H, Yatabe MS, Hashimoto S, Yoneda M, Felder RA, et al. Angiotensin II type 1 receptor blocker attenuates the activation of ERK and NADPH oxidase by mechanical strain in mesangial cells in the absence of angiotensin II. Am J Physiol Renal Physiol. 2009;296:F1052–60.
Acknowledgments
NIH/NIA AG29904 to MA and AG25056 to MFL. NIH/NIDDK DK61578 and Norman Hackerman Advanced Research Program, Texas, to TH.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Asghar, M., Tayebati, S.K., Lokhandwala, M.F. et al. Potential Dopamine-1 Receptor Stimulation in Hypertension Management. Curr Hypertens Rep 13, 294–302 (2011). https://doi.org/10.1007/s11906-011-0211-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-011-0211-1