Abstract
Background
PET-based radiomics features could predict the biological characteristics of primary prostate cancer (PCa). However, the optimal thresholds to predict the biological characteristics of PCa are unknown. This study aimed to compare the predictive power of 18F-PSMA-1007 PET radiomics features at different thresholds for predicting multiple biological characteristics.
Methods
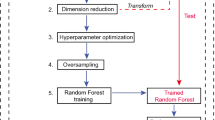
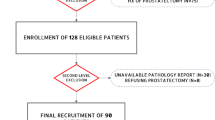
One hundred and seventy-three PCa patients with complete preoperative 18F-PSMA-1007 PET examination and clinical data before surgery were collected. The prostate lesions' volumes of interest were semi-automatically sketched with thresholds of 30%, 40%, 50%, and 60% maximum standardized uptake value (SUVmax). The radiomics features were respectively extracted. The prediction models of Gleason score (GS), extracapsular extension (ECE), and vascular invasion (VI) were established using the support vector machine. The performance of models from different thresholding regions was assessed using receiver operating characteristic curve and confusion matrix-derived indexes.
Results
For predicting GS, the 50% SUVmax model showed the best predictive performance in training (AUC, 0.82 [95%CI 0.74–0.88]) and testing cohorts (AUC, 0.80 [95%CI 0.66–0.90]). For predicting ECE, the 40% SUVmax model exhibit the best predictive performance (AUC, 0.77 [95%CI 0.68–0.84] and 0.77 [95%CI 0.63–0.88]). As for VI, the 50% SUVmax model had the best predictive performance (AUC, 0.74 [95%CI 0.65–0.82] and 0.74 [95%CI 0.56–0.82]).
Conclusion
The 18F-1007-PSMA PET-based radiomics features at 40–50% SUVmax showed the best predictive performance for multiple PCa biological characteristics evaluation. Compared to the single PSA model, radiomics features may provide additional benefits in predicting the biological characteristics of PCa.



Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Guglielmo P, Marturano F, Bettinelli A, Gregianin M, Paiusco M, Evangelista L (2021) Additional value of PET radiomic features for the initial staging of prostate cancer: a systematic review from the literature. Cancers (Basel). https://doi.org/10.3390/cancers13236026
Foley RW, Redman SL, Graham RN, Loughborough WW, Little D (2020) Fluorine-18 labelled prostate-specific membrane antigen (PSMA)-1007 positron-emission tomography-computed tomography: normal patterns, pearls, and pitfalls. Clin Radiol 75(12):903–913. https://doi.org/10.1016/j.crad.2020.06.031
Moussa AS, Li J, Soriano M, Klein EA, Dong F, Jones JS (2009) Prostate biopsy clinical and pathological variables that predict significant grading changes in patients with intermediate and high grade prostate cancer. BJU Int 103(1):43–48. https://doi.org/10.1111/j.1464-410X.2008.08059.x
Hoogland AM, Kweldam CF, van Leenders GJ (2014) Prognostic histopathological and molecular markers on prostate cancer needle-biopsies: a review. BioMed Res Int 2014:341324. https://doi.org/10.1155/2014/341324
D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ, Wein A (1998) Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280(11):969–974. https://doi.org/10.1001/jama.280.11.969
Jeong BC, Chalfin HJ, Lee SB, Feng Z, Epstein JI, Trock BJ, Partin AW, Humphreys E, Walsh PC, Han M (2015) The relationship between the extent of extraprostatic extension and survival following radical prostatectomy. Eur Urol 67(2):342–346. https://doi.org/10.1016/j.eururo.2014.06.015
Kwart AM, Sims JE (1978) Blood vascular invasion: a poor prognostic factor in adenocarcinoma of the prostate. J Urol 119(1):138–140. https://doi.org/10.1016/s0022-5347(17)57411-x
Egevad L, Delahunt B, Srigley JR, Samaratunga H (2016) International Society of Urological Pathology (ISUP) grading of prostate cancer—an ISUP consensus on contemporary grading. APMIS Acta Pathol Microbiol Immunol Scand 124(6):433–435. https://doi.org/10.1111/apm.12533
Serefoglu EC, Altinova S, Ugras NS, Akincioglu E, Asil E, Balbay MD (2013) How reliable is 12-core prostate biopsy procedure in the detection of prostate cancer? Can Urol Assoc J J l’Assoc Urol Can 7(5–6):E293-298. https://doi.org/10.5489/cuaj.11224
Czarniecki M, Mena E, Lindenberg L, Cacko M, Harmon S, Radtke JP, Giesel F, Turkbey B, Choyke PL (2018) Keeping up with the prostate-specific membrane antigens (PSMAs): an introduction to a new class of positron emission tomography (PET) imaging agents. Transl Androl Urol 7(5):831–843. https://doi.org/10.21037/tau.2018.08.03
Zippel C, Ronski SC, Bohnet-Joschko S, Giesel FL, Kopka K (2020) Current status of PSMA-Radiotracers for prostate cancer: data analysis of prospective trials listed on ClinicalTrials.gov. Pharmaceuticals (Basel, Switzerland) 13(1):12. https://doi.org/10.3390/ph13010012
Wester HJ, Schottelius M (2019) PSMA-targeted radiopharmaceuticals for imaging and therapy. Sem Nucl Med 49(4):302–312. https://doi.org/10.1053/j.semnuclmed.2019.02.008
Moazemi S, Erle A, Khurshid Z, Lutje S, Muders M, Essler M, Schultz T, Bundschuh RA (2021) Decision-support for treatment with (177)Lu-PSMA: machine learning predicts response with high accuracy based on PSMA-PET/CT and clinical parameters. Ann Transl Med 9(9):818. https://doi.org/10.21037/atm-20-6446
Thomas L, Kantz S, Hung A, Monaco D, Gaertner FC, Essler M, Strunk H, Laub W, Bundschuh RA (2018) (68)Ga-PSMA-PET/CT imaging of localized primary prostate cancer patients for intensity modulated radiation therapy treatment planning with integrated boost. Eur J Nucl Med Mol Imaging 45(7):1170–1178. https://doi.org/10.1007/s00259-018-3954-y
Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, Giesel F, Haberkorn U, Hope TA, Kopka K, Krause BJ, Mottaghy FM, Schoder H, Sunderland J, Wan S, Wester HJ, Fanti S, Herrmann K (2017) (68)Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging 44(6):1014–1024. https://doi.org/10.1007/s00259-017-3670-z
Sengupta S, Asha Krishnan M, Chattopadhyay S, Chelvam V (2019) Comparison of prostate-specific membrane antigen ligands in clinical translation research for diagnosis of prostate cancer. Cancer Rep (Hoboken, NJ) 2(4):e1169. https://doi.org/10.1002/cnr2.1169
Awenat S, Piccardo A, Carvoeiras P, Signore G, Giovanella L, Prior JO, Treglia G (2021) Diagnostic role of (18)F-PSMA-1007 PET/CT in prostate cancer staging: a systematic review. Diagnostics (Basel) 11(3):552. https://doi.org/10.3390/diagnostics11030552
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278(2):563–577. https://doi.org/10.1148/radiol.2015151169
Damascelli A, Gallivanone F, Cristel G, Cava C, Interlenghi M, Esposito A, Brembilla G, Briganti A, Montorsi F, Castiglioni I, De Cobelli F (2021) Advanced imaging analysis in prostate MRI: building a radiomic signature to predict tumor aggressiveness. Diagnostics (Basel) 11(4):594. https://doi.org/10.3390/diagnostics11040594
Papp L, Spielvogel CP, Grubmuller B, Grahovac M, Krajnc D, Ecsedi B, Sareshgi RAM, Mohamad D, Hamboeck M, Rausch I, Mitterhauser M, Wadsak W, Haug AR, Kenner L, Mazal P, Susani M, Hartenbach S, Baltzer P, Helbich TH, Kramer G, Shariat SF, Beyer T, Hartenbach M, Hacker M (2021) Supervised machine learning enables non-invasive lesion characterization in primary prostate cancer with [(68)Ga]Ga-PSMA-11 PET/MRI. Eur J Nucl Med Mol Imaging 48(6):1795–1805. https://doi.org/10.1007/s00259-020-05140-y
Solari EL, Gafita A, Schachoff S, Bogdanovic B, Villagran Asiares A, Amiel T, Hui W, Rauscher I, Visvikis D, Maurer T, Schwamborn K, Mustafa M, Weber W, Navab N, Eiber M, Hatt M, Nekolla SG (2022) The added value of PSMA PET/MR radiomics for prostate cancer staging. Eur J Nucl Med Mol Imaging 49(2):527–538. https://doi.org/10.1007/s00259-021-05430-z
Alongi P, Stefano A, Comelli A, Laudicella R, Scalisi S, Arnone G, Barone S, Spada M, Purpura P, Bartolotta TV, Midiri M, Lagalla R, Russo G (2021) Radiomics analysis of 18F-Choline PET/CT in the prediction of disease outcome in high-risk prostate cancer: an explorative study on machine learning feature classification in 94 patients. Eur Radiol 31(7):4595–4605. https://doi.org/10.1007/s00330-020-07617-8
Zamboglou C, Carles M, Fechter T, Kiefer S, Reichel K, Fassbender TF, Bronsert P, Koeber G, Schilling O, Ruf J, Werner M, Jilg CA, Baltas D, Mix M, Grosu AL (2019) Radiomic features from PSMA PET for non-invasive intraprostatic tumor discrimination and characterization in patients with intermediate- and high-risk prostate cancer—a comparison study with histology reference. Theranostics 9(9):2595–2605. https://doi.org/10.7150/thno.32376
Cysouw MCF, Jansen BHE, van de Brug T, Oprea-Lager DE, Pfaehler E, de Vries BM, van Moorselaar RJA, Hoekstra OS, Vis AN, Boellaard R (2021) Machine learning-based analysis of [(18)F]DCFPyL PET radiomics for risk stratification in primary prostate cancer. Eur J Nucl Med Mol Imaging 48(2):340–349. https://doi.org/10.1007/s00259-020-04971-z
Tu SJ, Tran VT, Teo JM, Chong WC, Tseng JR (2021) Utility of radiomic zones for risk classification and clinical outcome predictions using supervised machine learning during simultaneous (11) C-choline PET/MRI acquisition in prostate cancer patients. Med Phys 48(9):5192–5201. https://doi.org/10.1002/mp.15064
Giesel F, Sterzing F, Schlemmer H, Holland-Letz T, Mier W, Rius M, Afshar-Oromieh A, Kopka K, Debus J, Haberkorn U, Kratochwil C (2016) Intra-individual comparison of (68)Ga-PSMA-11-PET/CT and multi-parametric MR for imaging of primary prostate cancer. Eur J Nucl Med Mol Imaging 43(8):1400–1406. https://doi.org/10.1007/s00259-016-3346-0
Zamboglou C, Schiller F, Fechter T, Wieser G, Jilg CA, Chirindel A, Salman N, Drendel V, Werner M, Mix M, Meyer PT, Grosu AL (2016) (68)Ga-HBED-CC-PSMA PET/CT versus histopathology in primary localized prostate cancer: a voxel-wise comparison. Theranostics 6(10):1619–1628. https://doi.org/10.7150/thno.15344
Bettermann AS, Zamboglou C, Kiefer S, Jilg CA, Spohn S, Kranz-Rudolph J, Fassbender TF, Bronsert P, Nicolay NH, Gratzke C, Bock M, Ruf J, Benndorf M, Grosu AL (2019) [(68)Ga-]PSMA-11 PET/CT and multiparametric MRI for gross tumor volume delineation in a slice by slice analysis with whole mount histopathology as a reference standard—implications for focal radiotherapy planning in primary prostate cancer. Radiother Oncol 141:214–219. https://doi.org/10.1016/j.radonc.2019.07.005
Incerti E, Fodor A, Mapelli P, Fiorino C, Alongi P, Kirienko M, Giovacchini G, Busnardo E, Gianolli L, Di Muzio N, Picchio M (2015) Radiation treatment of lymph node recurrence from prostate cancer: Is 11C-choline PET/CT predictive of survival outcomes? J Nucl Med 56(12):1836–1842. https://doi.org/10.2967/jnumed.115.163741
Zwanenburg A, Leger S, Vallières M, Löck S, Initiative F (2016) Image biomarker standardisation initiative
Zamboglou C, Bettermann AS, Gratzke C, Mix M, Ruf J, Kiefer S, Jilg CA, Benndorf M, Spohn S, Fassbender TF, Bronsert P, Chen M, Guo H, Wang F, Qiu X, Grosu AL (2021) Uncovering the invisible-prevalence, characteristics, and radiomics feature-based detection of visually undetectable intraprostatic tumor lesions in (68)GaPSMA-11 PET images of patients with primary prostate cancer. Eur J Nucl Med Mol Imaging 48(6):1987–1997. https://doi.org/10.1007/s00259-020-05111-3
Spohn SKB, Kramer M, Kiefer S, Bronsert P, Sigle A, Schultze-Seemann W, Jilg CA, Sprave T, Ceci L, Fassbender TF, Nicolay NH, Ruf J, Grosu AL, Zamboglou C (2020) Comparison of manual and semi-automatic [(18)F]PSMA-1007 PET based contouring techniques for intraprostatic tumor delineation in patients with primary prostate cancer and validation with histopathology as standard of reference. Front Oncol 10:600690. https://doi.org/10.3389/fonc.2020.600690
Ding C, Peng H (2005) Minimum redundancy feature selection from microarray gene expression data. J Bioinform Comput Biol 3(2):185–205. https://doi.org/10.1142/s0219720005001004
Zhu D, Zhang M, Li Q, Liu J, Zhuang Y, Chen Q, Chen C, Xiang Y, Zhang Y, Yang Y (2021) Can perihaematomal radiomics features predict haematoma expansion? Clin Radiol 76(8):e621–e629. https://doi.org/10.1016/j.crad.2021.03.003
Horwich A, Parker C, de Reijke T, Kataja V, Group EGW (2013) Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(Suppl 6):106–114. https://doi.org/10.1093/annonc/mdt208
Fan X, Xie N, Chen J, Li T, Cao R, Yu H, He M, Wang Z, Wang Y, Liu H, Wang H, Yin X (2022) Multiparametric MRI and machine learning based radiomic models for preoperative prediction of multiple biological characteristics in prostate cancer. Front Oncol 12:839621. https://doi.org/10.3389/fonc.2022.839621
Mouraviev V, Villers A, Bostwick DG, Wheeler TM, Montironi R, Polascik TJ (2011) Understanding the pathological features of focality, grade and tumour volume of early-stage prostate cancer as a foundation for parenchyma-sparing prostate cancer therapies: active surveillance and focal targeted therapy. BJU Int 108(7):1074–1085. https://doi.org/10.1111/j.1464-410X.2010.10039.x
Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE (2016) The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur Urol 70(1):106–119. https://doi.org/10.1016/j.eururo.2016.02.028
Gleason DF (1992) Histologic grading of prostate cancer: a perspective. Hum Pathol 23(3):273–279. https://doi.org/10.1016/0046-8177(92)90108-f
Andrén O, Fall K, Franzén L, Andersson SO, Johansson JE, Rubin MA (2006) How well does the Gleason score predict prostate cancer death? A 20-year followup of a population based cohort in Sweden. J Urol 175(4):1337–1340. https://doi.org/10.1016/s0022-5347(05)00734-2
Stark JR, Perner S, Stampfer MJ, Sinnott JA, Finn S, Eisenstein AS, Ma J, Fiorentino M, Kurth T, Loda M, Giovannucci EL, Rubin MA, Mucci LA (2009) Gleason score and lethal prostate cancer: Does 3 + 4 = 4 + 3? J Clin Oncol 27(21):3459–3464. https://doi.org/10.1200/JCO.2008.20.4669
Hou Y, Zhang YH, Bao J, Bao ML, Yang G, Shi HB, Song Y, Zhang YD (2021) Artificial intelligence is a promising prospect for the detection of prostate cancer extracapsular extension with mpMRI: a two-center comparative study. Eur J Nucl Med Mol Imaging 48(12):3805–3816. https://doi.org/10.1007/s00259-021-05381-5
Seifert R, Herrmann K, Kleesiek J, Schafers M, Shah V, Xu Z, Chabin G, Grbic S, Spottiswoode B, Rahbar K (2020) Semiautomatically quantified tumor volume using (68)Ga-PSMA-11 PET as a biomarker for survival in patients with advanced prostate cancer. J Nucl Med 61(12):1786–1792. https://doi.org/10.2967/jnumed.120.242057
Mihatsch PW, Beissert M, Pomper MG, Bley TA, Seitz AK, Kubler H, Buck AK, Rowe SP, Serfling SE, Hartrampf PE, Werner RA (2022) Changing threshold-based segmentation has no relevant impact on semi-quantification in the context of structured reporting for PSMA-PET/CT. Cancers (Basel) 14(2):270. https://doi.org/10.3390/cancers14020270
Gafita A, Bieth M, Krönke M, Tetteh G, Navarro F, Wang H, Günther E, Menze B, Weber WA, Eiber M (2019) qPSMA: semiautomatic software for whole-body tumor burden assessment in prostate cancer using (68)Ga-PSMA11 PET/CT. J Nucl Med 60(9):1277–1283. https://doi.org/10.2967/jnumed.118.224055
Schmidkonz C, Cordes M, Schmidt D, Bäuerle T, Goetz TI, Beck M, Prante O, Cavallaro A, Uder M, Wullich B, Goebell P, Kuwert T, Ritt P (2018) (68)Ga-PSMA-11 PET/CT-derived metabolic parameters for determination of whole-body tumor burden and treatment response in prostate cancer. Eur J Nucl Med Mol Imaging 45(11):1862–1872. https://doi.org/10.1007/s00259-018-4042-z
Schmuck S, von Klot CA, Henkenberens C, Sohns JM, Christiansen H, Wester HJ, Ross TL, Bengel FM, Derlin T (2017) Initial experience with volumetric (68)Ga-PSMA I&T PET/CT for assessment of whole-body tumor burden as a quantitative imaging biomarker in patients with prostate cancer. J Nucl Med 58(12):1962–1968. https://doi.org/10.2967/jnumed.117.193581
Draulans C, De Roover R, van der Heide UA, Kerkmeijer L, Smeenk RJ, Pos F, Vogel WV, Nagarajah J, Janssen M, Isebaert S, Maes F, Mai C, Oyen R, Joniau S, Kunze-Busch M, Goffin K, Haustermans K (2021) Optimal (68)Ga-PSMA and (18)F-PSMA PET window levelling for gross tumour volume delineation in primary prostate cancer. Eur J Nucl Med Mol Imaging 48(4):1211–1218. https://doi.org/10.1007/s00259-020-05059-4
Chang AJ, Autio KA, Roach M 3rd, Scher HI (2014) High-risk prostate cancer-classification and therapy. Nat Rev Clin Oncol 11(6):308–323. https://doi.org/10.1038/nrclinonc.2014.68
Bangma CH, Roobol MJ (2012) Defining and predicting indolent and low risk prostate cancer. Crit Rev Oncol Hematol 83(2):235–241. https://doi.org/10.1016/j.critrevonc.2011.10.003
Howrey BT, Kuo YF, Lin YL, Goodwin JS (2013) The impact of PSA screening on prostate cancer mortality and overdiagnosis of prostate cancer in the United States. J Gerontol Ser A Biol Sci Med Sci 68(1):56–61. https://doi.org/10.1093/gerona/gls135
Funding
This study was supported by the Wenzhou Major Program of Science and Technology Innovation (Grant No. ZY2020012), the Health Foundation for Creative Talents in Zhejiang Province, China (Grant No. 2016), the Project Foundation for the College Young and Middle-aged Academic Leader of Zhejiang Province, China (Grant No. 2017), and Basic Research Project of Wenzhou (Grant No. Y2020164).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by SB, DZ, YY, KP, ZP, XF and KT. The first draft of the manuscript was written by FY and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All human studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of The First Affiliated Hospital of Wenzhou Medical University.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, F., Bian, S., Zhu, D. et al. Machine learning-based radiomics for multiple primary prostate cancer biological characteristics prediction with 18F-PSMA-1007 PET: comparison among different volume segmentation thresholds. Radiol med 127, 1170–1178 (2022). https://doi.org/10.1007/s11547-022-01541-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-022-01541-1