Abstract
Purpose
Methanotrophs are an important group of bacteria that can metabolize methane. Polycyclic aromatic hydrocarbons (PAHs) are widespread contaminants and present in all ecosystems. We hypothesize that PAHs may affect methanotrophs and methane oxidation. In this study, we assessed dose–response curves for the inhibition of methane oxidation and methanotrophs diversity by pyrene, and resistance and resilience of soil methane oxidation rate and methanotrophs composition in response to pyrene contamination.
Material and methods
Methanotrophic bacterial diversity was determined by terminal restriction fragment length polymorphism analysis of the pmoA gene, while methanotrophs composition was assessed by cloning and sequencing of the pmoA gene. Sequences with 98% or more identity were considered as the same operational taxonomic unit (OTU). The pyrene concentration at which methane oxidation decreased by 50%, as compared to the non-spiked control soil (EC50), was determined. Both EC50 concentration and 500 mg kg−1of pyrene were applied as disturbances in the resistance and resilience experiment. Resistance and resilience were determined 1 and 40 days, respectively, after spiking pyrene.
Results and discussion
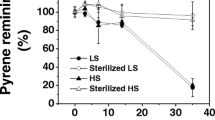
Methane oxidation rate decreased with increasing pyrene concentrations and the EC50 value was 22.0 mg kg−1. Methanotrophic bacterial community diversity decreased in 200 and 500 mg kg−1 pyrene treatments, and methanotroph community structure shifts occurred in 100, 200, and 500 mg kg−1 pyrene treatments. Methane oxidation was neither resistant nor resilient to pyrene disturbance. However, methane oxidation of soil with 22.0 mg kg−1 pyrene disturbance recovered to some extent after 40 days incubation. There were five OTUs identified in the control samples, but the number of OTUs increased 1 day after the addition of 22 mg kg−1 of pyrene. It suggests that a low level of disturbance may increase diversity. Forty days after 500 mg kg−1 of pyrene disturbance, only one OTU belonging to Methylocaldum was detected. The resilience of Methylocaldum to a high level of pyrene could be due to the high genomic GC content, which reduces the frequency of insertion by pyrene into the DNA duplex. In addition, we found that the number of OTUs decreased in all treatments after the 40-day incubation.
Conclusions
Methane oxidation activity was more sensitive to pyrene than the methanotroph community structure, but could recover under a low level of pyrene. Significant decrease in diversity and shift in species composition occurred only after severe perturbation. A low level of disturbance could increase biodiversity, while a high level of disturbance could decrease it.





Similar content being viewed by others
References
Allison G (2004) The influence of species diversity and stress intensity on community resistance and resilience. Ecol Monogr 74:117–134
Austin MP (1987) Models for the analyses of species’ response to environmental gradients. Vegetatio 69:35–45
Bakken LR (1997) Culturable and nonculturable bacteria in soil. In: van Elsas JD, Trevors JT, Wellington EMH (eds) Modern soil microbiology. Marcel Dekker, New York, pp 47–62
Bamborough L, Cummings SP (2009) The impact of increasing heavy metal stress on the diversity and structure of the bacterial and actinobacterial communities of metallophytic grassland soil. Biol Fertil Soils 45:273–280
Bharati K, Mohanty SR, Adhya TK, Banerjee A, Rao VR, Sethunathan N (1999) Influence of a commercial formulation of tridemorph on methane production and oxidation in a tropical rice soil. Chemosphere 39:933–943
Bodelier PLE, Laanbroek HJ (2004) Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol Ecol 47:265–277
Bodelier PLE, Roslev P, Henckel T, Frenzel P (2000) Stimulation by ammonium-based fertilizers of methane oxidation in soil around rice roots. Nature 403:421–424
Bowman J (2006) The methanotrophs—the families methylococcaceae and methylocystaceae. In: Dworkin MM, Schleifer KH, Rosenberg E, Falkow S (eds) Prokaryotes: a handbook on the biology of bacteria, vol 5: proteobacteria: alpha and beta subclasses, 3 rdth edn. Springer, New York, pp 266–289
Chan OC, Yang XD, Fu Y, Feng ZL, Sha LQ, Casper P, Zou XM (2006) 16S rRNA gene analyses of bacterial community structures in the soils of evergreen broadleaved forests in South-West China. FEMS Microbiol Ecol 58:247–259
Costello AM, Lidstrom ME (1999) Molecular characterization of functional and phylogenetic genes from natural populations of methanotrophs in lake sediments. Appl Environ Microbiol 65:5066–5074
Dedysh SN, Panikov NS, Liesack W, Grobkopf R, Zhou J, Tiedje JM (1998) Isolation of acidophilic methane-oxidizing bacteria from northern peat wetlands. Science 282:281–284
Dedysh SN, Liesack W, Khmelenina VN, Suzina NE, Trotsenko YA, Semrau JD, Bares AM, Panikov NS, Tiedje JM (2000) Methylocella palustris gen. nov., sp. nov., a new methane-oxidizing acidophilic bacterium from peat bogs representing a novel subtype of serine-pathway methanotrophs. Int J Syst Evol Microbiol 50:955–969
Deng H, Li XF, Cheng WD, Zhu YG (2009) Resistance and resilience of Cu-polluted soil after Cu perturbation, tested by a wide range of soil microbial parameters. FEMS Microbiol Ecol 70:293–304
Deng H, Zhang B, Yin R, Hl W, Mitchell SM, Griffiths BS, Daniell TJ (2010) Long-term effect of re-vegetation on the microbial community of a severely eroded soil in sub-tropical China. Plant Soil 328:447–458
Dong LH, Córdova-Kreylos AL, Yang JS, Yuan HL, Scow KM (2009) Humic acids buffer the effects of urea on soil ammonia oxidizers and potential nitrification. Soil Biol Biochem 41:1612–1621
Einola JKM, Kettunen RH, Rintala JA (2007) Responses of methane oxidation to temperature and water content in cover soil of a boreal landfill. Soil Biol Biochem 39:1156–1164
Gentry TJ, Wolf DC, Reynolds CM, Fuhrmann JJ (2003) Pyrene and phenanthrene influence on soil microbial populations. Soil Sediment Contam 7:53–68
Griffiths BS, Ritz K, Wheatley R, Kuan HL, Boag B, Christensen S, Ekelund F, Sorensen SJ, Muller S, Bloem J (2001) An examination of the biodiversity–ecosystem function relationship in arable soil microbial communities. Soil Biol Biochem 33:1713–1722
Griffiths RI, Whiteley AS, O’Donnell AG, Bailey MJ (2003) Physiological and community response of established grassland bacterial populations to water stress. Appl Environ Microbiol 69:6961–6968
Griffiths BS, Kuan HL, Ritz K, Glover LA, McCaig AE, Fenwick C (2004) The relationship between microbial community structure and functional stability, tested experimentally in an upland pasture soil. Microb Ecol 47:104–113
Grime JP (1973) Competitive exclusion in herbaceous vegetation. Nature 242:344–347
Grime JP (1979) Plant strategies and vegetation processes. Wiley, Chichester, p 222
Groot TT, VanBodegom PM, Harren FJM, Meijer HAJ (2003) Quantification of methane oxidation in the rice rhizosphere using 13C-labelled methane. Biogeochem 64:355–372
Haritash AK, Kaushik CP (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169:1–15
Hoffmann T, Horz HP, Kemnitz D, Conrad R (2002) Diversity of the particulate methane mono-oxygenase gene in methanotrophic samples from different rice field soils in China and the Philippines. Syst Appl Microbiol 25:267–274
Hütsch BW (1998) Methane oxidation in arable soil as inhibited by ammonium, nitrite, and organic manure with respect to soil pH. Biol Fertil Soils 28:27–35
Joseph SJ, Hugenholtz P, Sangwan P, Osborne CA, Janssen PH (2003) Laboratory cultivation of widespread and previously uncultured soil bacteria. Appl Environ Microbiol 69:7210–7215
Kassen R, Rainey PB (2004) The ecology and genetics of microbial diversity. Annu Rev Microbiol 58:207–231
Khan S, Aijun L, Zhang SZ, Zhu YG (2008) Accumulation of polycyclic aromatic hydrocarbons and heavy metals in lettuce grown in the soils contaminated with long-term wastewater irrigation. J Hazard Mater 152:506–515
Klimkowicz-Pawlas A, Maliszewska-Kordybach B (2003) Effect of anthracene and pyrene on dehydrogenases activity in soils exposed and unexposed to pahs. Water Air Soil Pollut 145:169–186
Knief C, Vanitchung S, Harvey NW, Conrad R, Dunfield PF, Chidthaisong A (2005) Diversity of methanotrophic bacteria in tropical upland soils under different land uses. Appl Environ Microbiol 71:3826–3831
Kozdroj J, van Elsas JD (2001) Structural diversity of microorganisms in chemically perturbed soil assessed by molecular and cytochemical approaches. J Microbiol Meth 43:197–212
Lee T, Park JW (2006) Recovery of iron reactivity for removal of Cr(VI) using iron-reducing consortium. KSCE J Civ Eng 10:175–180
Ling WT, Zeng YC, Cao YZ, Dang HJ, Zhu XZ (2010) Availability of polycyclic aromatic hydrocarbons in aging soils. J Soils Sediments 9:799–807
Luton PE, Wayne JM, Sharp RJ, Riley PW (2002) The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen populations in landfill. Microbiology 148:3521–3530
Maliszewska-Kordybach B (2005) Dissipation of polycyclic aromatic hydrocarbons in freshly contaminated soils—the effect of soil physicochemical properties and aging. Water Air Soil Pollut 168:113–128
Maliszewska-Kordybach B, Klimkowicz-Pawlas A, Smreczak B (2007) Ecotoxic effect of phenanthrene on nitrifying bacteria in soils of different properties. J Environ Qual 36:1635–1645
Miles RA, Doucette WJ (2001) Assessing the aerobic biodegradability of 14 hydrocarbons in two soils using a simple microcosm/respiration method. Chemosphere 45:1085–1090
Mohanty SR, Bharati K, Deepa N, Rao VR, Adhya TK (2000) Influence of heavy metals on methane oxidation in tropical rice soils. Ecotoxicol Environ Saf 47:277–284
Nälsund J, Hedman JE, Agestrand C (2008) Effects of the antibiotic ciprofloxacin on the bacterial community structure and degradation of pyrene in marine sediment. Aquat Toxicol 90:223–227
Noll M, Frenzel P, Conrad R (2008) Selective stimulation of type I methanotrophs in a rice paddy soil by urea fertilization revealed by RNA-based stable isotope probing. FEMS Microbiol Ecol 65:125–132
Park JR, Moon S, Ahn YM, Kim JY, Nam K (2005) Determination of environmental factors influencing methane oxidation in a sandy landfill cover soil. Environ Technol 26:93–102
Prieme A, Ekelund F (2001) Five pesticides decreased oxidation of atmospheric methane in a forest soil. Soil Biol Biochem 33:831–835
Prus-Glowacki W, Wojnicka-Poltorak A, Oleksyn J, Reich PB (1999) Industrial pollutants tend to increase genetic diversity: evidence from field-grown European scots pine populations. Water Air Soil Pollut 116:395–402
Rejmánková E, Rejmánek M, Djohan T, Goldman CR (1999) Resistance and resilience of subalpine wetlands With respect to prolonged drought. Folia Geobot 34:175–188
Rockne KJ, Stensel HD, Herwig RP, Strand SE (1998) PAH degradation and bioaugmentation by a Marine Methanotrophic enrichment. Biorem J 1:209–222
Sait M, Hugenholtz P, Janssen PH (2002) Cultivation of genetically distinct soil bacteria from phylogenetic lineages previously only detected in culture-independent surveys. Environ Microbiol 4:654–666
Shen GQ, Lu YT, Zhou QX, Hong JB (2005) Interaction of polycyclic aromatic hydrocarbons and heavy metals on soil enzyme. Chemosphere 61:1175–1182
Slater GF, Cowie BR, Harper N, Droppo IG (2008) Variation in PAH inputs and microbial community in surface sediments of Hamilton Harbour: implications to remediation and monitoring. Environ Pollut 153:60–70
Sooksa-nguan T, Thies JE, Gypmantasiri P, Boonkerd N, Teaumroong N (2009) Effect of rice cultivation systems on nitrogen cycling and nitrifying bacterial community structure. Appl Soil Ecol 43:139–149
Stralis-Pavese N, Sessitsch A, Weilharter A, Reichenauer T, Riesing J, Csontos J, Murrell JC, Bodrossy L (2004) Optimization of diagnostic microarray for application in analysing landfill methanotroph communities under different plant covers. Environ Microbiol 6:347–363
Tamai N, Takenaka C, Ishizuka S (2007) Water-soluble Al inhibits methane oxidation at atmospheric concentration levels in Japanese forest soil. Soil Biol Biochem 39:1730–1736
Tang JB, Liang SJ, Zhang JB, Gao ZQ, Zhang SH (2009) pGreen-S: a clone vector bearing absence of enhanced green fluorescent protein for screening recombinants. Anal Biochem 388:173–174
Thielemann T, Lücke A, Schleser GH, Littke R (2000) Methane exchange between coal-bearing basins and the atmosphere: the Ruhr Basin and the Lower Rhine Embayment, Germany. Org Geochem 31:1387–1408
Tiensing T, Preston S, Strachan N, Paton GI (2001) Soil solution extraction techniques for microbial ecotoxicity testing: a comparative evaluation. J Environ Monit 3:91–96
Trotsenko YA, Medvedkova KA, Khmelenina VN, BTs E (2009) Thermophilic and thermotolerant aerobic methanotrophs. Microbiology 78:387–401
Xu XK, Inubushi K (2009) Ethylene oxidation, atmospheric methane consumption, and ammonium oxidation in temperate volcanic forest soils. Biol Fertil Soils 45:265–271
Yan T, Zhou J, Zhang CL (2006) Diversity of functional genes for methanotrophs in sediments associated with gas hydrates and hydrocarbon seeps in the Gulf of Mexico. FEMS Microbiol Ecol 57:251–259
You SJ, Hsu CL, Ouyang CF (2002) Identification of the microbial diversity of wastewater nutrient removal processes using molecular biotechnology. Biotechnol Lett 24:1361–1366
Zhang B, Deng H, Hl W, Yin R, Hallett PD, Griffiths BS, Daniell TJ (2010) Does microbial habitat or community structure drive the functional stability of microbes to stresses following re-vegetation of a severely degraded soil? Soil Biol Biochem 42:850–859
Zheng Y, Zhang LM, Zheng YM, Di HJ, He JZ (2008) Abundance and community composition of methanotrophs in a Chinese paddy soil under long-term fertilization practices. J Soils Sediments 8:406–414
Acknowledgments
This project is supported by the Chinese Academy of Sciences (KZCX1-YW-06-03) and the Ministry of Science and Technology (2007CB407301, 2007CB407304).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ji-Zheng He
Rights and permissions
About this article
Cite this article
Deng, H., Guo, GX. & Zhu, YG. Pyrene effects on methanotroph community and methane oxidation rate, tested by dose–response experiment and resistance and resilience experiment. J Soils Sediments 11, 312–321 (2011). https://doi.org/10.1007/s11368-010-0306-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-010-0306-3