Abstract
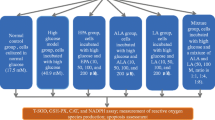
This study was aimed at elucidating the protective effects of 18β-glycyrrhetinic acid (18βGA) against acrylamide (Acr)-induced cellular damage in diabetic rats. Rats were randomly assigned into eight groups (n = 8) following 12 h of fasting: control group, a single dose of 50 mg/kg streptozotocin (STZ) intraperitoneally (diabetic group), 50 mg/kg 18βGA orally after 2 weeks from STZ injection (18βGA group), 20 mg/kg Acr after 1month from STZ injection (Acr group), STZ plus Acr (STZ-Acr group), STZ plus 18βGA (STZ-18βGA group), Acr plus 18βGA (Acr-18βGA group), or STZ plus Acr plus 18βGA (STZ-Acr-18βGA group). Administration of 18βGA alone increased GSH, GSH-PX, SOD, and CAT in both liver and kidneys. While STZ injection was associated with diabetic and oxidative stress changes as indicated by the higher serum glucose, cholesterol, creatinine, IL-1β, IL-6, TNF-α, and antioxidant enzyme activities, together with increased lipid peroxides and decreased antioxidant biomarkers in the liver and kidneys. Similarly, the co-administration of STZ and Acr was associated with similar, more augmented effects, compared to STZ alone. The administration of 18βGA normalized STZ and Acr-induced elevations in oxidative defense variables in the liver and kidney tissues and blood biomarkers. Thus, our study demonstrated that the damaging effects of Acr were more exaggerated in diabetic rats. Furthermore, it showed the ability of 18βGA to inhibit reactive oxygen species generation and restore the antioxidant defenses in diabetic rats with Acr-induced liver and kidney cytotoxicity.




Similar content being viewed by others
Data availability
All required data will be available from the corresponding author upon request.
References
Abdel-Daim MM, Abd Eldaim MA, Hassan AG (2015) Trigonella foenum-graecum ameliorates acrylamide-induced toxicity in rats: roles of oxidative stress, proinflammatory cytokines, and DNA damage. Biochem Cell Biol 93(3):192–198. https://doi.org/10.1139/bcb-2014-0122
Abdel-Daim MM, Abo El-Ela FI, Alshahrani FK, Bin-Jumah M, Al-Zharani M, Almutairi B, Alyousif MS, Bungau S, Aleya L, Alkahtani S (2020) Protective effects of thymoquinone against acrylamide-induced liver, kidney and brain oxidative damage in rats. Environ Sci Pollut Res 27(30):37709–37717. https://doi.org/10.1007/s11356-020-09516-3
Adewale O, Brimson J, Odunola O, Gbadegesin M, Owumi S, Isidoro C, Tencomnao T (2015) The potential for plant derivatives against acrylamide neurotoxicity. Phytother Res 29(7):978–985
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahmed MM, Laila I, Ibrahim M (2018) Rutin ameliorates acrylamide-induced hepatotoxicity and biochemical disturbance in male albino rats. Scientific Journal of October 6 University 4(2):8–13
Al Deeb S, Al Moutaery K, Arshaduddin M, Biary N, Tariq M (2000) Attenuation of acrylamide-induced neurotoxicity in diabetic rats. Neurotoxicol Teratol 22(2):247–253
Aleissa MS, Alkahtani S, Abd Eldaim MA, Ahmed AM, Bungău SG, Almutairi B, Bin-Jumah M, AlKahtane AA, Alyousif MS, Abdel-Daim MM (2020) Fucoidan ameliorates oxidative stress, inflammation, DNA damage, and hepatorenal injuries in diabetic rats intoxicated with aflatoxin B1. Oxidative Med Cell Longev 2020:9316751–9316710. https://doi.org/10.1155/2020/9316751
Anwar MM, Meki A-RM (2003) Oxidative stress in streptozotocin-induced diabetic rats: effects of garlic oil and melatonin. Comp Biochem Physiol A Mol Integr Physiol 135(4):539–547
Babson SR, Babson AL (1973) An improved amylase assay using dyed amylopectin. Clin Chim Acta 44(2):193–197 0009-8981(73)90381-1 [pii]
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Bin-Jumah MN, Al-Huqail AA, Abdelnaeim N, Kamel M, Fouda MMA, Abulmeaty MMA, Saadeldin IM, Abdel-Daim MM (2021) Potential protective effects of Spirulina platensis on liver, kidney, and brain acrylamide toxicity in rats. Environ Sci Pollut Res Int 28:26653–26663. https://doi.org/10.1007/s11356-021-12422-x
Chu P, Lin L, Chen P, Su T, Lin C (2017) Negative association between acrylamide exposure and body composition in adults: NHANES, 2003–2004. Nutrition & diabetes 7(3):e246–e246
Costa LG, Aschner M, Vitalone A, Syversen T, Soldin OP (2004) Developmental neuropathology of environmental agents. Annu Rev Pharmacol Toxicol 44:87–110
Coulombe JJ, Favreau L (1963) A new simple semimicro method for colorimetric determination of urea. Clin Chem 9(1):102–108
Dandona P, Aljada A, Bandyopadhyay A (2004) Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 25(1):4–7
Dias A, Porawski M, Alonso M, Marroni N, Collado P, González-Gallego J (2005) Quercetin decreases oxidative stress, NF-κB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J Nutr 135:2299–2304
Domingueti CP, Dusse LMSA, das Graças Carvalho M, de Sousa LP, Gomes KB, Fernandes AP (2016) Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complicat 30(4):738–745
Elhelaly AE, AlBasher G, Alfarraj S, Almeer R, Bahbah EI, Fouda MMA, Bungău SG, Aleya L, Abdel-Daim MM (2019) Protective effects of hesperidin and diosmin against acrylamide-induced liver, kidney, and brain oxidative damage in rats. Environ Sci Pollut Res 26(34):35151–35162. https://doi.org/10.1007/s11356-019-06660-3
El-Saber Batiha G, Magdy Beshbishy A, El-Mleeh A, Abdel-Daim MM, Prasad Devkota H (2020) Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 10(3). https://doi.org/10.3390/biom10030352
Ge B, Yang D, Wu X, Zhu J, Wei W, Yang B (2018) Cytoprotective effects of glycyrrhetinic acid liposome against cyclophosphamide-induced cystitis through inhibiting inflammatory stress. Int Immunopharmacol 54:139–144
Gheibi S, Kashfi K, Ghasemi A (2017) A practical guide for induction of type-2 diabetes in rat: incorporating a high-fat diet and streptozotocin. Biomed Pharmacother 95:605–613
Graebin CS (2018) The pharmacological activities of glycyrrhizinic acid (“glycyrrhizin”) and glycyrrhetinic acid. In: Mérillon JM, Ramawat K (eds) Sweeteners. Reference Series in Phytochemistry. Springer, Cham. https://doi.org/10.1007/978-3-319-27027-2_15
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126(1):131–138 0003-2697(82)90118-X [pii]
Gumpricht E, Dahl R, Devereaux MW, Sokol RJ (2005) Licorice compounds glycyrrhizin and 18β-glycyrrhetinic acid are potent modulators of bile acid-induced cytotoxicity in rat hepatocytes. J Biol Chem 280(11):10556–10563
Hsu F-L, Liu I-M, Kuo D-H, Chen W-C, Su H-C, Cheng J-T (2003) Antihyperglycemic effect of puerarin in streptozotocin-induced diabetic rats. J Nat Prod 66(6):788–792
Kalaiarasi P, Kaviarasan K, Pugalendi KV (2009) Hypolipidemic activity of 18β-glycyrrhetinic acid on streptozotocin-induced diabetic rats. Eur J Pharmacol 612(1-3):93–97
Khursheed R, Singh SK, Wadhwa S, Kapoor B, Gulati M, Kumar R, Ramanunny AK, Awasthi A, Dua K (2019) Treatment strategies against diabetes: success so far and challenges ahead. Eur J Pharmacol 862:172625. https://doi.org/10.1016/j.ejphar.2019.172625
Kind P, King E (1954) Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol 7(4):322–326
Larsen K (1972) Creatinine assay by a reaction-kinetic principle. Clin Chim Acta 41:209–217
Lin C-Y, Lin Y-C, Kuo H-K, Hwang J-J, Lin J-L, Chen P-C, Lin L-Y (2009) Association among acrylamide, blood insulin, and insulin resistance in adults. Diabetes Care 32(12):2206–2211
Liu WJ, Xie SH, Liu YN, Kim W, Jin HY, Park SK, Shao YM, Park TS (2012) Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin-induced diabetic rats. J Pharmacol Exp Ther 340(2):248–255
Mahmoud AM, Al Dera HS (2015) 18β-Glycyrrhetinic acid exerts protective effects against cyclophosphamide-induced hepatotoxicity: potential role of PPARγ and Nrf2 upregulation. Genes Nutr 10(6):1–13
Mahmoud AM, Hussein OE, Hozayen WG, Abd El-Twab SM (2017) Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of PPARγ and Nrf2: protective effect of 18β-Glycyrrhetinic acid. Chem Biol Interact 270:59–72. https://doi.org/10.1016/j.cbi.2017.04.009
Mehri S, Karami HV, Hassani FV, Hosseinzadeh H (2014) Chrysin reduced acrylamide-induced neurotoxicity in both in vitro and in vivo assessments. Iran Biomed J 18(2):101–106
Mihara M, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86(1):271–278
Montilla PL, Vargas JF, Túnez IF, Carmen M, de Agueda M, Valdelvira MED, Cabrera ES (1998) Oxidative stress in diabetic rats induced by streptozotocin: protective effects of melatonin. J Pineal Res 25(2):94–100
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46(2):849–854
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169 0022-2143(67)90076-5 [pii]
Pastorino G, Cornara L, Soares S, Rodrigues F, Oliveira MBP (2018) Liquorice (Glycyrrhiza glabra): a phytochemical and pharmacological review. Phytother Res 32(12):2323–2339
Podell BK, Ackart DF, Richardson MA, DiLisio JE, Pulford B, Basaraba RJ (2017) A model of type 2 diabetes in the guinea pig using sequential diet-induced glucose intolerance and streptozotocin treatment. Dis Model Mech 10(2):151–162
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1):56–63
Singh S, Gamlath S, Wakeling L (2007) Nutritional aspects of food extrusion: a review. Int J Food Sci Technol 42(8):916–929
Smith C, Perfetti T, Garg R, Hansch C (2003) IARC carcinogens reported in cigarette mainstream smoke and their calculated log P values. Food Chem Toxicol 41(6):807–817
Tomaszewska E, Dobrowolski P, Puzio I, Prost L, Kurlak P, Sawczuk P, Badzian B, Hulas-Stasiak M, Kostro K (2014) Acrylamide-induced prenatal programming of intestine structure in guinea pig. J Physiol Pharmacol 65(1):107–115
Venkatasubbaiah K, Venkataswamy D, Suresh K, Rao K (2014) Acrylamide induced oxidative stress in rat and chick embtyonic liver. Indo Am J Pharmac Res 6:2791–2798
Xie X, Lu C, Wu M, Liang J, Ying Y, Liu K, Huang X, Zheng S, Du X, Liu D, Wen Z, Hao G, Yang G, Feng L, Jing C (2020) Association between triclocarban and triclosan exposures and the risks of type 2 diabetes mellitus and impaired glucose tolerance in the National Health and Nutrition Examination Survey (NHANES 2013–2014). Environ Int 136:105445. https://doi.org/10.1016/j.envint.2019.105445
Yang T-H (2008) Recent applications of polyacrylamide as biomaterials. Recent Pat Mater Sci 1(1):29–40
Yin G, Liao S, Gong D, Qiu H (2021) Association of acrylamide and glycidamide haemoglobin adduct levels with diabetes mellitus in the general population. Environ Pollut 277:116816. https://doi.org/10.1016/j.envpol.2021.116816
Zhang Y, Zhang Y (2007) Formation and reduction of acrylamide in Maillard reaction: a review based on the current state of knowledge. Crit Rev Food Sci Nutr 47(5):521–542
Zhu F, Cai YZ, Ke J, Corke H (2009) Evaluation of the effect of plant extracts and phenolic compounds on reduction of acrylamide in an asparagine/glucose model system by RP-HPLC-DAD. J Sci Food Agric 89(10):1674–1681
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research, University of Hafr Al Batin for funding this work through the project No G-202-2020.
Author information
Authors and Affiliations
Contributions
Idea and protocol design: ISA, SA, and MM A-D
Methodology and experimentation; ISA, ME, ME, SA, and MM A-D
Data analysis: ME, ME, and MM A-D
Funding: ISA, SA, and MM A-D
All authors shared draft writing; all authors approved the submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This is an animal study. Our experimental protocol was approved by the Animal Care and Ethics Review Committee at the Faculty of Veterinary Medicine, Suez Canal University, Egypt (approval no. 2020042).
Consent for publication
All authors approved this submission.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alanazi, I.S., Emam, M., Elsabagh, M. et al. The protective effects of 18β-glycyrrhetinic acid against acrylamide-induced cellular damage in diabetic rats. Environ Sci Pollut Res 28, 58322–58330 (2021). https://doi.org/10.1007/s11356-021-14742-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14742-4