Abstract
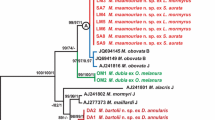
Two new species of Contracaecum Railliet & Henry, 1912, previously referred to as C. multipapillatum sp. A and C. multipapillatum sp. B by Nascetti et al. (1990) from the Dalmatian pelican Pelecanus crispus (L.) in the Ambracian Gulf off Greece, are described as C. gibsoni n. sp. and C. overstreeti n. sp., respectively. Morphological analysis and the differential diagnosis of genetically recognised male specimens of C. gibsoni and C. overstreeti with respect to C. multipapillatum (von Drasche, 1882) (sensu lato) from Egretta alba (L.) in northern Colombia and other morphologically related Contracaecum spp. enabled the detection of differences between the two species in a number of characters, including spicule length and the shape of its tip, and the arrangement of the proximal and distal papillae on the male tail. Accordingly, formal descriptions are presented for C. gibsoni n. sp. and C. overstreeti n. sp. from P. crispus. The genetic characterisation of the two taxa is based on 20 allozyme loci and sequence analyses (519 bp) of the mtDNA cox2 gene. Reproductive isolation was demonstrated between these two taxa, which sympatrically infect the same definitive host, and fixed allele differences between the two species were found at some (Aat-2, PepC-1, PepC-2 and Pgm-1) of the 20 allozyme loci analysed. Their genetic divergence, estimated at the allozyme level, was D Nei = 0.31. The genetic relationships of C. gibsoni and C. overstreeti with respect to C. multipapillatum (s. l.) collected from E. alba in Colombia, as well as with other congeners from fish-eating birds which had previously been genetically characterised using the same genetic markers, i.e. C. rudolphii A and C. rudolphii B of Bullini et al. (1986), C. septentrionale Kreis, 1955, C. microcephalum (Rudolphi, 1809), C. bioccai Mattiucci et al., 2008, C. pelagicum Johnston & Mawson, 1942 and C. micropapillatum (Stossich, 1890), were inferred from mtDNA cox2 sequence analysis. The MP, NJ and BI trees obtained were congruent in depicting C. gibsoni and C. overstreeti as closely related species but quite distinct from each other and forming a subclade with specimens of C. multipapilllatum (s. l.) from E. alba (L.) in Colombia. This subclade was also found to be distinct from the remainder of the taxa considered.




Similar content being viewed by others
References
Baylis, H. A., & Daubney, R. (1922). Report on the parasitic nematodes in the collection of the Zoological Survey of India. Memoirs of the Indian Museum, Calcutta, 7, 266–347.
Bullini, L., Nascetti, G., Paggi, L., Orecchia, P., Mattiucci, S., & Berland, B. (1986). Genetic variation of ascaridoid worms with different life cycles. Evolution, 40, 437–440.
Cavalli-Sforza, L. L., & Edwards, A. W. F. (1967). Phylogenetic analysis: models and estimation procedures. American Journal of Human Genetics, 19, 233–257.
Connal, A. (1912). Some nematode worms from Lagos. Journal of the London School of Tropical Medicine, 1, 229–237.
Courtney, C. H., & Forrester, D. (1974). Helminth parasites of the brown pelican in Florida and Louisiana. Proceedings of the Helminthological Society of Washington, 41, 89–93.
D’Amelio, S., Barros, N. B., Ingrosso, S., Fauquier, D. A., Russo, R., & Paggi, L. (2007). Genetic characterization of members of the genus Contracaecum (Nematoda: Anisakidae) from fish-eating birds from west-central Florida, USA, with evidence of new species. Parasitology, 134, 1041–1051.
Deardorff, T. L., & Overstreet, R. M. (1980). Contracaecum multipapillatum (synonym = C. robustum) from fishes and birds in the northern Gulf of Mexico. Journal of Parasitology, 66, 853–856.
Deardorff, T. L., & Overstreet, R. M. (1981). Terranova ceticola n. sp. (Nematoda: Anisakidae) from the dwarf sperm whale, Kogia simus (Owen), in the Gulf of Mexico. Systematic Parasitology, 3, 25–28.
Dronen, N. O., Blend, C. K., & Anderson, C. K. (2003). Endohelminths from the brown pelican, Pelecanus occidentalis, and the American white pelican, Pelicanus eythrorhynchus, from Galveston Bay, Texas, USA, and check list of pelican parasites. Comparative Parasitology, 70, 140–154.
Dyer, W. G., Williams, E. H., Mignucci-Giannoni, A. A., Jimenez-Marrero, N. M., Bunkley-Williams, L., Moore, D. P., et al. (2002). Helminth and arthropod parasites of the brown pelican, Pelecanus occidentalis, in Puerto Rico, with a compilation of all metazoan parasites reported from this host in the Western Hemisphere. Avian Pathology, 31, 441–448.
Fagerholm, H. P. (1989). Intra-specific variability of the morphology in a single population of the seal parasite Contracaecum osculatum (Rudolphi) (Nematoda, Ascaridoidea), with a redescription of the species. Zoologica Scripta, 18, 33–41.
Fagerholm, H. P. (1991). Systematic implications of male caudal morphology in ascaridoid nematode parasites. Systematic Parasitology, 19, 215–228.
Felsenstein, J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution, 39, 783–791.
Flores-Barroeta, L. (1957). Nematodes de aves y mamiferos. Revista Iberica de Parasitologia, 17, 277–297.
Hills, D. M., & Bull, J. J. (1993). An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology, 42, 182–192.
Huelsenbeck, J. P., & Ronquist, F. (2001). MrBayes: Bayesian inference of phylogeny. Bioinformatics, 17, 754–755.
Huizinga, H. W. (1967). Studies on the life cycle and development of Contracaecum spiculigerum (Rudolphi, 1809) (Ascaridoidea: Heterocheilidae) from marine piscivorous birds. Journal of the Elisa Mitchell Scientific Society, 82, 180–195.
Kreis, H. A. (1955). Contracaecum septentrionale, ein neuer parasit aus dem Kormoran; sein Labenslauf, sowie Angaben über die Entwicklung der Anisakinae. Zeitschrift fur Parasitenkünde, 17, 106–112.
Larget, B., & Simon, L. D. (1999). Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Molecular Biology and Evolution, 16, 750–759.
Li, A., D’Amelio, S., Paggi, L., He, F., Gasser, R. B., Lun, Z., et al. (2005). Genetic evidence for the existence of sibling species within Contracaecum rudolphii (Hartwich, 1964) and the validity of Contracaecum septentrionale (Kreis, 1955) (Nematoda: Anisakidae). Parasitology Research, 96, 361–366.
Lucker, J. T. (1941). A redescription of Contracaecum multipapillatum (von Drasche, 1882). Journal of Parasitology, 27, 505–512.
Mattiucci, S., Cianchi, R., Nascetti, G., Paggi, L., Sardella, N., Timi, J., et al. (2003). Genetic evidence for two sibling species within Contracaecum ogmorhini Johnston and Mawson (1941) (Nematoda: Anisakidae) from otariid seals of boreal and austral regions. Systematic Parasitology, 54, 13–23.
Mattiucci, S., & Nascetti, G. (2007). Genetic diversity and infection levels of anisakid nematodes parasitic in fish and marine mammals from Boreal and Austral hemispheres. Veterinary Parasitology, 148, 43–57.
Mattiucci, S., & Nascetti, G. (2008). Advances and trends in the molecular systematics of anisakid nematodes, with implications for their evolutionary ecology and host-parasite co-evolutionary processes. Advances in Parasitology, 66, 47–148.
Mattiucci, S., Nascetti, G., Cianchi, R., Paggi, L., Arduino, P., Margolis, L., et al. (1997). Genetic and ecological data on the Anisakis simplex complex with evidence for a new species (Nematoda, Ascaridoidea, Anisakidae). Journal of Parasitology, 83, 401–416.
Mattiucci, S., Nascetti, G., Dailey, M., Webb, S. C., Barros, N., Cianchi, R., et al. (2005). Evidence for a new species of Anisakis Dujardin, 1845: morphological description and genetic relationships between congeners (Nematoda: Anisakidae). Systematic Parasitology, 61, 157–171.
Mattiucci, S., Paoletti, M., Olivero-Verbel, J., Arrollo, B., Baldiris, R., & Nascetti, G. (2006). Evidence for new species of Contracaecum Railliet et Henry, 1912 (Nematoda, Anisakidae), parasites of fish-eating birds from Colombia: genetic relationships between congeners, and larval identification. Parassitologia, 48, 351.
Mattiucci, S., Paoletti, M., Olivero-Verbel, J., Baldiris, R., Arroyo-Salgado, B., Garbin, L., et al. (2008a). Contracaecum bioccai n. sp. from the brown pelican Pelecanus occidentalis (L.) in Colombia (Nematoda: Anisakidae): morphology, molecular evidence and its genetic relationship with congeners from fish-eating birds. Systematic Parasitology, 69, 101–121.
Mattiucci, S., Paoletti, M., Webb, S. C., Sardella, N., Timi, J. T., Berland, B., et al. (2008b). Genetic relationships among species of Contracaecum Railliet & Henry, 1912 and Phocascaris Höst, 1932 (Nematoda: Anisakidae) from pinnipeds inferred from mitochondrial cox2 sequences, and congruence with allozyme data. Parasite, 15, 408–419.
Moravec, F. (1994). Parasitic nematodes of freshwater fishes of Europe. Dordrecht: Kluwer Academic Publishers, 473 pp.
Nadler, S. A., D’Amelio, S., Dailey, M. D., Paggi, L., Siu, S., & Sakanari, J. A. (2005). Molecular phylogenetics and diagnosis of Anisakis, Pseudoterranova, and Contracaecum from northern Pacific marine mammals. Journal of Parasitology, 91, 1413–1429.
Nadler, S. A., D’Amelio, S., Fagerholm, H. P., Berland, B., & Paggi, L. (2000). Phylogenetic relationships among species of Contracaecum Railliet & Henry, 1912 and Phocascaris Host, 1932 (Nematoda: Ascaridoidea) based on nuclear rDNA sequence data. Parasitology, 121, 455–463.
Nadler, S. A., & Hudspeth, D. S. S. (2000). Phylogeny of the Ascaridoidea (Nematoda: Ascaridida) based on three genes and morphology: hypotheses of structural and sequence evolution. Journal of Parasitology, 86, 380–393.
Nascetti, G., Bullini, L., Cianchi, R., Paggi, L., Orecchia, P., Mattiucci, S., et al. (1990). Genetic relationships among anisakid species belonging to the genera Contracaecum and Phocascaris. Bulletin de la Société Française de Parasitologie, 8, 261.
Nascetti, G., Cianchi, R., Mattiucci, S., D’Amelio, S., Orecchia, P., Paggi, L., et al. (1993). Three sibling species within Contracaecum osculatum (Nematoda, Ascaridida, Ascaridoidea) from the Atlantic Arctic-boreal region: reproductive isolation and host preferences. International Journal for Parasitology, 23, 105–120.
Navone, G. T., Etchegoin, J. A., & Cremonte, F. (2000). Contracaecum multipapillatum (Nematoda: Anisakidae) from Egretta alba (Aves: Ardeidae) and comments on other species of this genus in Argentina. Journal of Parasitology, 86, 807–810.
Nei, M. (1972). Genetic distance between populations. American Naturalist, 106, 283–292.
Olivero-Verbel, J., Baldiris-Ávila, R., & Arroyo-Salgado, B. (2005). Nematode infection in Mugil incilis (Lisa) from Cartagena Bay and Totumo marsh, north of Colombia. Journal of Parasitology, 91, 1109–1112.
Olivero-Verbel, J., Baldiris-Ávila, R., Güette-Fernández, J., Benavides-Alvarez, A., Mercado-Camargo, J., & Arroyo-Salgado, B. (2006). Contracaecum sp. infection in Hoplias malabaricus (moncholo) from rivers and marshes of Colombia. Veterinary Parasitology, 140, 90–97.
Padgett, K. A., Nadler, S. A., Munson, L., Sacks, B., & Boyce, W. M. (2005). Systematics of Mesocestoides (Cestoda: Mesocestodidae): evaluation of molecular and morphological variation among isolates. Journal of Parasitology, 91, 1435–1443.
Paggi, L., Mattiucci, S., Gibson, D. I., Berland, B., Nascetti, G., Cianchi, R., et al. (2000). Pseudoterranova decipiens species A and B (Nematoda: Ascaridoidea): nomenclatural designation, morphological diagnostic characters and genetic markers. Systematic Parasitology, 45, 185–197.
Posada, D., & Buckley, T. R. (2004). Model selection and model averaging in phylogenetics: advantages of the AIC and Bayesian approaches over likelihood ratio tests. Systematic Biology, 53, 793–808.
Posada, D., & Crandall, K. A. (1998). Modeltest: testing the model of DNA substitution. Bioinformatics, 1, 817–818.
Pyrovetsi, M. (1997). Integrated management to create new breeding habitat for Dalmatian pelican (Pelecanus crispus) in Greece. Environmental Management, 21, 657–667.
Pyrovetsi, M., & Papazahariodou, M. (1995). Mortality factors of Dalmatian pelicans (Pelecanus crispus) wintering in Macedonia, Greece. Environmental Conservation, 22, 345–351.
Reeder, T. W. (2003). A phylogeny of the Australian Sphenomorphus group (Scincidae: Squamata) and the phylogenetic placement of the crocodile skinks (Tribolonotus): Bayesian approaches to assessing congruence and obtaining confidence in maximum likelihood inferred relationships. Molecular Phylogenetics and Evolution, 27, 384–397.
Shamsi, S., Gasser, R., Beveridge, I., & Shabani, A. A. (2008). Contracaecum pyripapillatum n. sp. (Nematoda: Anisakidae) and a description of C. multipapillatum (von Drasche, 1882) from the Australian pelican. Parasitology Research, 103, 1031–1039.
Shamsi, S., Norman, R., Gasser, R., & Beveridge, I. (2009a). Redescription and genetic characterization of selected Contracaecum spp. (Nematoda: Anisakidae) from various hosts in Australia. Parasitology Research, 104, 1507–1525.
Shamsi, S., Norman, R., Gasser, R., & Beveridge, I. (2009b). Genetic and morphological evidences for the existence of sibling species within Contracaecum rudolphii (Hartwich, 1964) (Nematoda: Anisakidae) in Australia. Parasitology Research, 105, 529–538.
Swofford, D. L. (2003). PAUP*. Phylogenetic analysis using parsimony (*and other methods). Sunderland, MA: Sinauer Associates.
Swofford, D. L., & Selander, R. B. A. (1997). Biosys 2. A computer program for the analysis of allelic variation genetics. Urbana, IL: University of Illinois.
Tamura, K., Dudley, J., Nei, M., & Kumar, S. (2007). MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24, 1596–1599.
Thompson, J. D., Higgins, D. G., & Gibson, T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weigh matrix choice. Nucleic Acids Research, 22, 4673–4680.
Valentini, A., Mattiucci, S., Bondanelli, P., Webb, S. C., Mignucci-Giannone, A., Colom-Llavina, M. M., et al. (2006). Genetic relationships among Anisakis species (Nematoda: Anisakidae) inferred from mitochondrial cox-2 sequences, and comparison with allozyme data. Journal of Parasitology, 92, 156–166.
Vevers, G. M. (1923). Report on the Entozoa collected from animals which died in the Zoological Gardens of London during eight months of 1919–1920. Proceedings of the Zoological Society of London, 3, 405–410.
Vidal-Martínez, V. M., Osorio-Sarabia, D., & Overstreet, R. M. (1994). Experimental infection of Contracaecum multipapillatum (Nematoda: Anisakinae) from Mexico in the domestic cat. Journal of Parasitology, 80, 576–579.
Zhu, X. Q., D’Amelio, S., Gasser, R. B., Yang, T. B., Paggi, L., He, F., et al. (2007). Practical PCR tools for the delineation of Contracaecum rudolphii A and Contracaecum rudolphii B (Ascaridoidea: Anisakidae) using genetic markers in nuclear ribosomal DNA. Molecular and Cellular Probes, 21, 97–102.
Acknowledgements
We thank Prof. M. Pyrovetsi (Department of Ecology, Aristotle University, Thessalononiki, Greece) for his kind collaboration and information on the Dalmatian pelican. We thank two anonymous referees for their useful reviews. This research was partly funded by grants from the I Faculty of Medicine of ‘Sapienza – University of Rome’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mattiucci, S., Paoletti, M., Solorzano, A.C. et al. Contracaecum gibsoni n. sp. and C. overstreeti n. sp. (Nematoda: Anisakidae) from the Dalmatian pelican Pelecanus crispus (L.) in Greek waters: genetic and morphological evidence. Syst Parasitol 75, 207–224 (2010). https://doi.org/10.1007/s11230-009-9220-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-009-9220-8