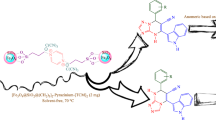
Abstract
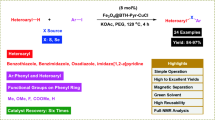
Magnetic separable nanoparticles-decorated N-heterocyclic carbene complex with copper (MNP[1-Methyl benzimidazole]NHC@Cu) has been prepared by covalent grafting of ionic liquid like 1-methyl benzimidazole unit on the surface of chlorofunctionalized Fe3O4 magnetic nanoparticles (MNPs) followed by metallation with copper(I) iodide. MNP[1-Methyl benzimidazole]NHC@Cu complex has been characterized by different techniques including Fourier transform infrared (FT-IR) spectroscopy, thermogravimetric analysis (TGA), energy-dispersive X-ray (EDX) analysis, X-ray diffraction (XRD), transmission electron microscopy (TEM) and vibrating sample magnetometer (VSM). MNP[1-Methyl benzimidazole]NHC@Cu complex was successfully implemented as heterogeneous catalyst in one-pot multicomponent synthesis of N-sulfonyl amidines from phenylacetylene, tosyl azide and amines at room temperature. Complex could be recycled six times without significant loss in the yield of product.
Graphic abstract











Similar content being viewed by others
References
S.C. Sau, P.K. Hota, S.K. Mandal, M. Soleilhavoup, G. Bertrand, Chem. Soc. Rev. 49, 1233 (2020)
D.M. Flanigan, F. Romanov-Michailidis, N.A. White, T. Rovis, Chem. Rev. 115, 9307 (2015)
J. Cao, M. Yu, L. Hanzhu Li, X. Wang, G. Zhu, Y. Wang, C. Cao. Shi, Res. Chem. Intermed. 41, 5323 (2015)
H. Diaz-Velazquez, F. Verpoort, Chem. Soc. Rev. 41, 7032e (2012)
W.A. Herrmann, Angew. Chem. Int. Ed. 41, 1290 (2002)
M.N. Hopkinson, C. Richter, M. Schedler, F. Glorius, Nature 510, 485 (2014)
D. Janssen-Muller, C. Schlepphorst, F. Glorius, Chem Soc Rev. 46, 4845 (2017)
S. Diez-Gonzalez, S.P. Nolan, Coord. Chem. Rev. 251, 874 (2007)
H.V. Huynh, Chem. Rev. 118, 9457 (2018)
W.J. Sommer, M. Weck, Coord. Chem. Rev. 251, 860 (2007)
H.J. Huang, W.C. Lee, G.P.A. Yap, T.G. Ong, J. Organomet. Chem. 761, 64 (2014)
R. Dorta, E.D. Stevens, N.M. Scott, C. Costabile, L. Cavallo, C.D. Hoff, S.P. Nolan, J. Am. Chem. Soc. 127(8), 2485 (2005)
G. Guisado-Barrios, J. Bouffard, B. Donnadieu, G. Bertrand, Angew. Chem. Int. Ed. Engl. 49, 4759 (2010)
C.S.J. Cazin, C. R. Chim. 12, 1173 (2009)
R. Zhong, A.C. Lindhorst, F.J. Groche, F. Kuhn, Chem. Rev. 117, 1970 (2017)
S. Gajare, A. Patil, S. Hangirgekar, S. Dhanmane, G. Rashinkar, Res. Chem. Intermed. 46, 2417 (2020)
M. Jagadale, S. Khanapure, R. Salunkhe, M. Rajmane, G. Rashinkar, Appl. Organomet. Chem. 30, 125 (2016)
A.R. Hajipour, N.S. Tadayoni, Z. Khorsandi, Appl. Organomet. Chem. 30, 590 (2016)
R.B. Nasir Baig, R.S. Varma, Chem. Commun. 49, 752 (2013)
R.K. Sharma, S. Dutta, S. Sharma, R. Zboril, R.S. Varma, M.B. Gawande, Green Chem. 18, 3184 (2016)
P. Wang, A.G. Kong, W.J. Wang, H.Y. Zhu, Y.K. Shan, Catal Lett. 135, 159 (2010)
J. Yuan, Y. Xu, A.H.E. Muller, Chem Soc Rev. 40, 640 (2011)
M.B. Gawande, P.S. Branco, R.S. Varma, Chem Soc Rev. 42, 3371 (2013)
M.Y. Lee, M.H. Kim, J. Kim, S.H. Kim, B.T. Kim, I.H. Jeong, S. Chang, S.H. Kim, S.-Y. Chang, Bioorg. Med. Chem. Lett. 20, 541 (2010)
P. Sienkiewich, K. Bielawski, A. Bielawska, J. Palka, Environ. Toxicol. Pharmacol. 20, 118 (2005)
J. Barker, M. Kilner, Coord. Chem. Rev. 133, 219 (1994)
Z. Xixi, L. Yunyun, W. Jie-Ping, Chin. J. Org. Chem. 40, 1891 (2020)
Z. Xixi, W. Jie-Ping, Adv. Synth. Catal. 361, 5690 (2019)
D. Mishra, A.J. Borah, P. Phukan, D. Hazarika, P. Phukan, Chem. Commun. 56, 8408 (2020)
F. Lazreg, M. Vasseur, A.M.Z. Slawin, C.S.J. Cazin, Beilstein J. Org. Chem. 16, 482 (2020)
X. He, Y. Shang, J. Hu, K. Ju, W. Jiang, S. Wang, Sci. China Chem. 55, 214 (2012)
L.M. Fleury, E.E. Wilson, M. Vogt, T.J. Fan, A.G. Oliver, B.L. Ashfeld, Angew. Chem. Int. Ed. 52, 11589 (2013)
K.A. Dekorver, W.L. Johnson, Y. Zhang, R.P. Hsung, H. Dai, J. Deng, A.G. Lohse, Y.S. Zhang, J. Org. Chem. 76, 5092 (2011)
S. Chen, Y. Xu, X. Wan, Org. Lett. 13, 6152 (2011)
R. Kurane, J. Jadhav, S. Khanapure, R. Salunkhe, G. Rashinkar, Green Chem. 15, 1849 (2013)
S. Khanapure, M. Jagadale, R. Salunkhe, G. Rashinkar, Res. Chem. Intermed. 42, 2075 (2016)
S. Patil, P.B. Pawar, S.D. Jadhav, M.B. Deshmukh, Asian J. Chem. 25, 9442 (2013)
S. Patil, S.D. Jadhav, M.B. Deshmukh, Arch. Appl. Sci. Res. 3, 203 (2011)
A. Patil, A. Mane, S. Kamat, T. Lohar, R. Salunkhe, Res. Chem. Intermed. 45, 3441 (2019)
X. Zhao, Y. Shi, T. Wang, Y. Cai, G. Jiang, J. Chomatogr A. 1188, 140 (2008)
Q. Liu, Z. Xu, J.A. Finch, R. Egerton. Chem Mater. 10, 3936 (1998)
M. Kooti, M. Afshari, Mater Res Bull. 47, 3473 (2012)
M.A. Zolfigol, R. Ayazi-Nasrabadi, RSC Adv. 6, 69595 (2016)
A. Salamatmanesh, M.K. Miraki, E. Yazdani, A. Heydari, Catal. Lett. 148, 3257 (2018)
M. Pellei, V. Gandin, C. Marzano, M. Marinelli, F.D. Bello, C. Santini, Appl. Organomet. Chem. 32, e4185 (2018)
M.K. Miraki, M. Arefi, A. Salamatmanesh, E. Yazdani, A. Heydari, Cat. Lett. 148, 3378 (2018)
A. Ying, H. Hou, S. Liu, G. Chen, J. Yang, S. Xu, A.C.S. Sustain, Chem. Eng. 4, 625 (2016)
S. Ghosh, A.Z.M. Badruddoza, M.S. Uddin, K. Hidajat, J Colloid Interface Sci. 354, 483 (2011)
J. Kim, S.S. Stahl, J. Org. Chem. 80, 2448 (2015)
M. Jagadale, P. Bhange, R. Salunkhe, D. Bhange, M. Rajmane, G. Rashinkar, Appl Catal A-Gen. 211, 95 (2016)
S.H. Kim, S.H. Park, J.H. Choi, S. Chang, Chem. Asian J. 6, 2618 (2011)
I. Bae, H. Han, S. Chang, J. Am. Chem. Soc. 27, 2038 (2005)
T. Yang, H. Cui, C. Zhang, L. Zhang, C.-Y. Su, Inorg. Chem. 52, 9053 (2013)
S.-J. Hwang, S.H. Cho, S.-B. Chang, Pure Appl. Chem. 80, 873 (2008)
E.J. Yoo, M. Ahlquist, I. Bae, K.B. Sharpless, V.V. Fokin, S. Chang, J. Org. Chem. 73, 5520 (2008)
M.J. Kim, B.R. Kim, C.Y. Lee, J. Kim, Tetrahedron Lett. 57, 4070 (2016)
Acknowledgements
We gratefully acknowledge Indian Institute of Bombay (IITB), North-Estern Hill University Shillong (NEHU), Indian Institute of Technology, Madras (IITM), for providing spectral facilities and Central Facility Centre (CFC) Shivaji University Kolhapur for providing quantitative spectral analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pawar, A., Gajare, S., Patil, A. et al. One-pot multicomponent synthesis of N-sulfonyl amidines using magnetic separable nanoparticles-decorated N-heterocyclic carbene complex with copper. Res Chem Intermed 47, 2801–2820 (2021). https://doi.org/10.1007/s11164-021-04455-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-021-04455-1