Abstract
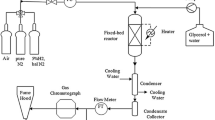
Kinetic modeling of the hydrogenolysis of glycerol was performed; a chromium-free Ni–Cu–SiO2 nanocomposite catalyst was used to produce 1,2-propanediol (1,2-PDO) at a low hydrogen-to-glycerol ratio. Kinetic data were produced in a fixed-bed reactor under a variety of temperatures, hydrogen-to-glycerol ratios, and space velocities. The reaction rates were developed based on the two-step reaction mechanism (the production of 1,2-PDO via acetol) and the reaction pathways for the byproducts observed in the experimental study. The kinetic parameters were estimated by fitting kinetic data, and the validity of the developed model was corroborated. Further analysis using the model showed that the reaction at a high temperature and a low space velocity could accomplish 95% conversion of glycerol; However, the low temperature was preferred to minimize the production of unwanted species (intermediate and byproducts) at the slight expense of conversion.



Similar content being viewed by others
Data availability
The datasets in the main manuscript will be presented upon request.
Abbreviations
- A t :
-
Cross-sectional area of the catalytic tube, cm2
- c i :
-
Concentration of species i, mmol/cm3
- C P :
-
Heat capacity, J/(g·K)
- C WP :
-
Dimensionless Weisz-Prater parameter
- D t :
-
Tube diameter, cm
- E :
-
Activation energy, J/mol
- F obj :
-
Objective function
- Δ H :
-
Heat of adsorption, J/mol
- Δ H r :
-
Heat of reaction, J/mol
- k :
-
Forward reaction rate constant
- K :
-
Adsorption equilibrium constant, bar–1
- \(\dot{m}\) :
-
Mass flow rate, g/s
- NE :
-
Number of experimental conditions
- NR :
-
Number of reactions
- P :
-
Partial pressure, bar
- R i :
-
Reaction rate, mmol/(gcat·h)
- T :
-
Temperature, K
- U :
-
Overall heat transfer coefficient, W/(m2·K)
- w i :
-
Weighting factor
- calc:
-
Calculated values
- exp:
-
Experimental data
- ref:
-
Reference
- W :
-
Wall
- \(\rho_{B}\) :
-
Bulk density, g/cm3
References
Balaraju M, Rekha V, Prasad PSS, Devi BLAP, Prasad RBN, Lingaiah N (2009) Influence of solid acids as co-catalysts on glycerol hydrogenolysis to propylene glycol over Ru/C catalysts. Appl Catal A: Gen 354(1):82–87. https://doi.org/10.1016/j.apcata.2008.11.010
Lipinsky ES, Sinclair RG (1986) Is lactic acid a commodity chemical? Chem Eng Prog 82(8):26–32
Pagliaro M, Ciriminna R, Kimura H, Rossi M, Della Pina C (2007) From glycerol to value-added products. Angew Chem Int Ed 46(24):4434–4440. https://doi.org/10.1002/anie.200604694
Wang Y, Zhou J, Guo X (2015) Catalytic hydrogenolysis of glycerol to propanediols: a review. RSC Adv 5(91):74611–74628. https://doi.org/10.1039/C5RA11957J
Akiyama M, Sato S, Takahashi R, Inui K, Yokota M (2009) Dehydration–hydrogenation of glycerol into 1,2-propanediol at ambient hydrogen pressure. Appl Catal A: Gen 371(1):60–66. https://doi.org/10.1016/j.apcata.2009.09.029
Bienholz A, Hofmann H, Claus P (2011) Selective hydrogenolysis of glycerol over copper catalysts both in liquid and vapour phase: Correlation between the copper surface area and the catalyst’s activity. Appl Catal A: Gen 391(1):153–157. https://doi.org/10.1016/j.apcata.2010.08.047
Kwak BK, Park DS, Yun YS, Yi J (2012) Preparation and characterization of nanocrystalline CuAl2O4 spinel catalysts by sol–gel method for the hydrogenolysis of glycerol. Catal Commun 24:90–95. https://doi.org/10.1016/j.catcom.2012.03.029
Priya SS, Kumar VP, Kantam ML, Bhargava SK, Periasamy S, Chary KVR (2015) Metal–acid bifunctional catalysts for selective hydrogenolysis of glycerol under atmospheric pressure: a highly selective route to produce propanols. Appl Catal A: Gen 498:88–98. https://doi.org/10.1016/j.apcata.2015.03.025
Vanama PK, Kumar A, Ginjupalli SR, Komandur VRC (2015) Vapor-phase hydrogenolysis of glycerol over nanostructured Ru/MCM-41 catalysts. Catal Today 250:226–238. https://doi.org/10.1016/j.cattod.2014.03.036
Dasari MA, Kiatsimkul P-P, Sutterlin WR, Suppes GJ (2005) Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl Catal A: Gen 281(1):225–231. https://doi.org/10.1016/j.apcata.2004.11.033
Huang Z, Cui F, Kang H, Chen J, Zhang X, Xia C (2008) Highly dispersed silica-supported copper nanoparticles prepared by precipitation—gel method: a simple but efficient and stable catalyst for glycerol hydrogenolysis. Chem Mater 20(15):5090–5099. https://doi.org/10.1021/cm8006233
Pandey DK, Pandhare NN, Biswas P (2019) Production of propylene glycol (propane-1,2-diol) in vapor phase over Cu–Ni/γ-Al2O3 catalyst in a down flow tubular reactor: effect of catalyst calcination temperature and kinetic study. React Kinet Mech Catal 127(1):523–542. https://doi.org/10.1007/s11144-019-01582-0
Meena ML, Malviya H, Pandhare NN, Biswas P (2022) Kinetic modeling of conversion of glycerol to 1,2-propanediol over bifunctional LDH catalyst. Curr Res Green Sustain Chem 5:100289. https://doi.org/10.1016/j.crgsc.2022.100289
Zhou Z, Li X, Zeng T, Hong W, Cheng Z, Yuan W (2010) Kinetics of hydrogenolysis of glycerol to propylene glycol over Cu–ZnO–Al2O3 catalysts. Chin J Chem Eng 18(3):384–390. https://doi.org/10.1016/S1004-9541(10)60235-2
Sharma RV, Kumar P, Dalai AK (2014) Selective hydrogenolysis of glycerol to propylene glycol by using Cu:Zn:Cr: Zr mixed metal oxides catalyst. Appl Catal A: Gen 477:147–156. https://doi.org/10.1016/j.apcata.2014.03.007
Pandhare NN, Pudi SM, Mondal S, Pareta K, Kumar M, Biswas P (2018) Development of kinetic model for hydrogenolysis of glycerol over Cu/MgO catalyst in a slurry reactor. Ind Eng Chem Res 57(1):101–110. https://doi.org/10.1021/acs.iecr.7b03684
Mondal S, Biswas P (2022) Conversion of bio-glycerol to propylene glycol over basic oxides (MgO, La2O3, MgO–La2O3, CaO, and BaO2) supported Cu–Zn bimetallic catalyst: a reaction kinetic study. Environ Technol Innov 27:102367. https://doi.org/10.1016/j.eti.2022.102367
Lee M, Hwang YK, Chang J-S, Chae H-J, Hwang DW (2016) Vapor-phase hydrogenolysis of glycerol to 1,2-propanediol using a chromium-free Ni–Cu–SiO2 nanocomposite catalyst. Catal Commun 84:5–10. https://doi.org/10.1016/j.catcom.2016.05.022
Fogler HS (2010) Essentials of chemical reaction engineering. Pearson Education, London
Yfanti VL, Ipsakis D, Lemonidou AA (2018) Kinetic study of liquid phase glycerol hydrodeoxygenation under inert conditions over a Cu-based catalyst. Reac Kinet Mech Cat 3(4):559–571. https://doi.org/10.1039/C8RE00061A
Mondal S, Malviya H, Biswas P (2019) Kinetic modelling for the hydrogenolysis of bio-glycerol in the presence of a highly selective Cu–Ni–Al2O3 catalyst in a slurry reactor. Reac Kinet Mech Cat 4(3):595–609. https://doi.org/10.1039/C8RE00138C
Acknowledgements
This work was supported by the Low-Carbon Chemical Technology Innovation Program for Alternative Fuel from Petroleum (Grant No. RS-2022-00156196) funded by the Ministry of Trade, Industry, and Energy (MOTIE), Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Woo, Y., Lee, M., Cha, S.H. et al. Kinetic modeling of hydrogenolysis of glycerol to 1,2-propanediol using a chromium-free Ni–Cu–SiO2 nanocomposite catalyst. Reac Kinet Mech Cat 136, 867–879 (2023). https://doi.org/10.1007/s11144-023-02390-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02390-3