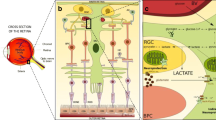
Abstract
In retina, like in brain, lactate equilibrates across cell membranes via monocarboxylate transporters and in the extracellular space by diffusion, forming a basis for the action of lactate as a transmitter of metabolic signals. In the present paper, we argue that the lactate receptor GPR81, also known as HCAR1, may contribute importantly to the control of retinal cell functions in health and disease. GPR81, a G-protein coupled receptor, is known to downregulate cAMP both in adipose and nervous tissue. The receptor also acts through other down-stream mechanisms to control functions, such as excitability, metabolism and inflammation. Recent publications predict effects of the lactate receptor on neurodegeneration. Neurodegenerative diseases in retina, where the retinal ganglion cells die, notably glaucoma and diabetic retinopathy, may be linked to disturbed lactate homeostasis. Pilot studies reveal high GPR81 mRNA in retina and indicate GPR81 localization in Müller cells and retinal ganglion cells. Moreover, monocarboxylate transporters are expressed in retinal cells. We envision that lactate receptors and transporters could be useful future targets of novel therapeutic strategies to protect neurons and prevent or counteract glaucoma as well as other retinal diseases.


Similar content being viewed by others
Abbreviations
- BRB:
-
Blood-retina barrier
- cAMP:
-
Adenosine 3′5′-cyclic monophosphate (cyclic AMP)
- CNS:
-
Central nervous system
- EAAT:
-
Excitatory amino acid transporter
- GPR81:
-
G-protein coupled receptor 81
- HCAR1:
-
Hydroxycarboxylic acid receptor 1
- MCTs:
-
Monocarboxylate transporters
References
Quigley HA, Broman AT (2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90:262–267
Antonetti DA, Klein R, Gardner TW (2012) Diabetic retinopathy. N Engl J Med 366:1227–1239
Vujosevic S, Midena E (2013) Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Muller cells alterations. J Diabetes Res 2013:905058
You Y, Gupta VK, Li JC, Klistorner A, Graham SL (2013) Optic neuropathies: characteristic features and mechanisms of retinal ganglion cell loss. Rev Neurosci 24:301–321
Lauritzen KH, Morland C, Puchades M, Holm-Hansen S, Hagelin EM, Lauritzen F, Attramadal H, Storm-Mathisen J, Gjedde A, Bergersen LH (2014) Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb Cortex 24:2784–2795
Diabetes, Data and Statistics. http://Www.Euro.Who.Int/En/Health-Topics/Noncommunicable-Diseases/Diabetes/Data-and-Statistics. Accessed 23 March 2015
Flammer J, Orgul S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, Renard JP, Stefansson E (2002) The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 21:359–393
Toft-Kehler AK, Skytt DM, Poulsen KA, Braendstrup CT, Gegelashvili G, Waagepetersen H, Kolko M (2014) Limited energy supply in Muller cells alters glutamate uptake. Neurochem Res 39:941–949
Hashimoto T, Hussien R, Cho HS, Kaufer D, Brooks GA (2008) Evidence for the mitochondrial lactate oxidation complex in rat neurons: demonstration of an essential component of brain lactate shuttles. PLoS ONE 3:e2915
Rinholm JE, Hamilton NB, Kessaris N, Richardson WD, Bergersen LH, Attwell D (2011) Regulation of oligodendrocyte development and myelination by glucose and lactate. J Neurosci 31:538–548
Schurr A, Payne RS, Miller JJ, Tseng MT, Rigor BM (2001) Blockade of lactate transport exacerbates delayed neuronal damage in a rat model of cerebral ischemia. Brain Res 895:268–272
Ross JM, Oberg J, Brene S, Coppotelli G, Terzioglu M, Pernold K, Goiny M, Sitnikov R, Kehr J, Trifunovic A, Larsson NG, Hoffer BJ, Olson L (2010) High brain lactate is a hallmark of aging and caused by a shift in the lactate dehydrogenase A/B ratio. Proc Natl Acad Sci USA 107:20087–20092
Lauritzen F, Eid T, Bergersen LH (2015) Monocarboxylate transporters in temporal lobe epilepsy: roles of lactate and ketogenic diet. Brain Struct Funct 220:1–12
Provis JM (2001) Development of the primate retinal vasculature. Prog Retin Eye Res 20:799–821
Mariga ST, Kolko M, Gjedde A, Bergersen LH (2014) Lactate transport and receptor actions in cerebral malaria. Front Neurosci 8:125
Wang L, Tornquist P, Bill A (1997) Glucose metabolism in pig outer retina in light and darkness. Acta Physiol Scand 160:75–81
Hurley JB, Lindsay KJ, Du J (2015) Glucose, lactate, and shuttling of metabolites in vertebrate retinas. J Neurosci Res 93:1079–1092
Ng SK, Wood JP, Chidlow G, Han G, Kittipassorn T, Peet DJ, Casson RJ (2015) Cancer-like metabolism of the mammalian retina. Clin Experiment Ophthalmol 43:367–376
Ait-Ali N, Fridlich R, Millet-Puel G, Clerin E, Delalande F, Jaillard C, Blond F, Perrocheau L, Reichman S, Byrne LC, Olivier-Bandini A, Bellalou J, Moyse E, Bouillaud F, Nicol X, Dalkara D, van Dorsselaer A, Sahel JA, Leveillard T (2015) Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell 161:817–832
Hill J, Rom S, Ramirez SH, Persidsky Y (2014) Emerging roles of pericytes in the regulation of the neurovascular unit in health and disease. J Neuroimmune Pharmacol 9:591–605
Bergersen L, Johannsson E, Veruki ML, Nagelhus EA, Halestrap A, Sejersted OM, Ottersen OP (1999) Cellular and subcellular expression of monocarboxylate transporters in the pigment epithelium and retina of the rat. Neuroscience 90:319–331
Kennedy-Lydon TM, Crawford C, Wildman SS, Peppiatt-Wildman CM (2013) Renal pericytes: regulators of medullary blood flow. Acta Physiol (Oxf) 207:212–225
Sakagami K, Wu DM, Puro DG (1999) Physiology of rat retinal pericytes: modulation of ion channel activity by serum-derived molecules. J Physiol 521(Pt 3):637–650
Chidlow G, Wood JP, Graham M, Osborne NN (2005) Expression of monocarboxylate transporters in rat ocular tissues. Am J Physiol Cell Physiol 288:C416–C428
Bergersen LH (2007) Is lactate food for neurons? Comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience 145:11–19
Chertov AO, Holzhausen L, Kuok IT, Couron D, Parker E, Linton JD, Sadilek M, Sweet IR, Hurley JB (2011) Roles of glucose in photoreceptor survival. J Biol Chem 286:34700–34711
Mergenthaler P, Lindauer U, Dienel GA, Meisel A (2013) Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci 36:587–597
Herzog RI, Jiang L, Herman P, Zhao C, Sanganahalli BG, Mason GF, Hyder F, Rothman DL, Sherwin RS, Behar KL (2013) Lactate preserves neuronal metabolism and function following antecedent recurrent hypoglycemia. J Clin Invest 123:1988–1998
Poitry-Yamate CL, Poitry S, Tsacopoulos M (1995) Lactate released by Muller glial cells is metabolized by photoreceptors from mammalian retina. J Neurosci 15:5179–5191
Winkler BS, Arnold MJ, Brassell MA, Puro DG (2000) Energy metabolism in human retinal Muller cells. Invest Ophthalmol Vis Sci 41:3183–3190
Dienel GA (2012) Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab 32:1107–1138
Dienel GA (2013) Astrocytic energetics during excitatory neurotransmission: What are contributions of glutamate oxidation and glycolysis? Neurochem Int 63:244–258
Lindsay KJ, Du J, Sloat SR, Contreras L, Linton JD, Turner SJ, Sadilek M, Satrustegui J, Hurley JB (2014) Pyruvate kinase and aspartate-glutamate carrier distributions reveal key metabolic links between neurons and glia in retina. Proc Natl Acad Sci USA 111:15579–15584
Goldman SS (1988) Gluconeogenesis in the amphibian retina. Lactate is preferred to glutamate as the gluconeogenic precursor. Biochem J 254:359–365
Mamczur P, Mazurek J, Rakus D (2010) Ubiquitous presence of gluconeogenic regulatory enzyme, fructose-1,6-bisphosphatase, within layers of rat retina. Cell Tissue Res 341:213–221
Skeie JM, Mahajan VB (2013) Proteomic interactions in the mouse vitreous-retina complex. PLoS ONE 8:e82140
Dienel GA, Cruz NF (2004) Nutrition during brain activation: Does cell-to-cell lactate shuttling contribute significantly to sweet and sour food for thought? Neurochem Int 45:321–351
Winkler BS, Pourcho RG, Starnes C, Slocum J, Slocum N (2003) Metabolic mapping in mammalian retina: a biochemical and 3H-2-deoxyglucose autoradiographic study. Exp Eye Res 77:327–337
Perezleon JA, Osorio-Paz I, Francois L, Salceda R (2013) Immunohistochemical localization of glycogen synthase and GSK3beta: control of glycogen content in retina. Neurochem Res 38:1063–1069
DiNuzzo M, Mangia S, Maraviglia B, Giove F (2013) Regulatory mechanisms for glycogenolysis and K+ uptake in brain astrocytes. Neurochem Int 63:458–464
Dienel GA, Cruz NF (2015) Contributions of glycogen to astrocytic energetics during brain activation. Metab Brain Dis 30:281–298
Dienel GA (2012) Fueling and imaging brain activation. ASN Neuro. doi:10.1042/AN20120021
Dienel GA (2015) The metabolic trinity, glucose-glycogen-lactate, links astrocytes and neurons in brain energetics, signaling, memory, and gene expression. Neurosci Lett. doi:10.1016/j.neulet.2015.02.052
Ghazi H, Osborne NN (1989) Agonist-induced glycogenolysis in rabbit retinal slices and cultures. Br J Pharmacol 96:895–905
Halestrap AP, Price NT (1999) The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J 343(Pt 2):281–299
Pierre K, Pellerin L (2005) Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem 94:1–14
Gerhart DZ, Leino RL, Drewes LR (1999) Distribution of monocarboxylate transporters MCT1 and MCT2 in rat retina. Neuroscience 92:367–375
Martin PM, Dun Y, Mysona B, Ananth S, Roon P, Smith SB, Ganapathy V (2007) Expression of the sodium-coupled monocarboxylate transporters SMCT1 (SLC5A8) and SMCT2 (SLC5A12) in retina. Invest Ophthalmol Vis Sci 48:3356–3363
Philp NJ, Wang D, Yoon H, Hjelmeland LM (2003) Polarized expression of monocarboxylate transporters in human retinal pigment epithelium and ARPE-19 cells. Invest Ophthalmol Vis Sci 44:1716–1721
Philp NJ, Ochrietor JD, Rudoy C, Muramatsu T, Linser PJ (2003) Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Invest Ophthalmol Vis Sci 44:1305–1311
Bergersen LH (2015) Lactate transport and signaling in the brain: potential therapeutic targets and roles in body–brain interaction. J Cereb Blood Flow Metab 35:176–185
Adijanto J, Du J, Moffat C, Seifert EL, Hurle JB, Philp NJ (2014) The retinal pigment epithelium utilizes fatty acids for ketogenesis. J Biol Chem 289:20570–20582
Barros LF (2013) Metabolic signaling by lactate in the brain. Trends Neurosci 36:396–404
Morland C, Lauritzen KH, Puchades M, Holm-Hansen S, Andersson K, Gjedde A, Attramadal H, Storm-Mathisen J, Bergersen LH (2015) The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: expression and action in brain. J Neurosci Res 93:1045–1055
Cureton EL, Kwan RO, Dozier KC, Sadjadi J, Pal JD, Victorino GP (2010) A different view of lactate in trauma patients: protecting the injured brain. J Surg Res 159:468–473
Bouzat P, Magistretti PJ, Oddo M (2014) Hypertonic lactate and the injured brain: facts and the potential for positive clinical implications. Intensive Care Med 40:920–921
Dienel GA (2014) Lactate shuttling and lactate use as fuel after traumatic brain injury: metabolic considerations. J Cereb Blood Flow Metab 34:1736–1748
Ahmed K, Tunaru S, Offermanns S (2009) GPR109A, GPR109B and GPR81, a family of hydroxy-carboxylic acid receptors. Trends Pharmacol Sci 30:557–562
Matschinsky FM, Passonneau JV, Lowry OH (1968) Quantitative histochemical analysis of glycolytic intermediates and cofactors with an oil well technique. J Histochem Cytochem 16:29–39
Berkowitz BA, Bansal N, Wilson CA (1994) Non-invasive measurement of steady-state vitreous lactate concentration. NMR Biomed 7:263–268
Cai TQ, Ren N, Jin L, Cheng K, Kash S, Chen R, Wright SD, Taggart AK, Waters MG (2008) Role of GPR81 in lactate-mediated reduction of adipose lipolysis. Biochem Biophys Res Commun 377:987–991
Liu C, Wu J, Zhu J, Kuei C, Yu J, Shelton J, Sutton SW, Li X, Yun SJ, Mirzadegan T, Mazur C, Kamme F, Lovenberg TW (2009) Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J Biol Chem 284:2811–2822
Feingold KR, Moser A, Shigenaga JK, Grunfeld C (2011) Inflammation inhibits GPR81 expression in adipose tissue. Inflamm Res 60:991–995
Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ (2014) Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 146:1763–1774
Franceschi C, Campisi J (2014) Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69(Suppl 1):S4–S9
Boehm MR, Oellers P, Thanos S (2011) Inflammation and immunology of the vitreoretinal compartment. Inflamm Allergy Drug Targets 10:283–309
Parmeggiani F, Romano MR, Costagliola C, Semeraro F, Incorvaia C, D’Angelo S, Perri P, De Palma P, De Nadai K, Sebastiani A (2012) Mechanism of inflammation in age-related macular degeneration. Mediators Inflamm 2012:546786
Lind KR, Ball KK, Cruz NF, Dienel GA (2013) The unfolded protein response to endoplasmic reticulum stress in cultured astrocytes and rat brain during experimental diabetes. Neurochem Int 62:784–795
Pavan B, Capuzzo A, Forlani G (2014) High glucose-induced barrier impairment of human retinal pigment epithelium is ameliorated by treatment with Goji berry extracts through modulation of cAMP levels. Exp Eye Res 120:50–54
Rutecki PA (1995) Noradrenergic modulation of epileptiform activity in the hippocampus. Epilepsy Res 20:125–136
Arnsten AF, Wang M, Paspalas CD (2015) Dopamine’s actions in primate prefrontal cortex: challenges for treating cognitive disorders. Pharmacol Rev 67:681–696
Whitaker CM, Cooper NG (2010) Differential distribution of exchange proteins directly activated by cyclic AMP within the adult rat retina. Neuroscience 165:955–967
Bozzo L, Puyal J, Chatton JY (2013) Lactate modulates the activity of primary cortical neurons through a receptor-mediated pathway. PLoS ONE 8:e71721
Newman EA (2013) Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature. J Cereb Blood Flow Metab 33:1685–1695
Peppiatt CM, Howarth C, Mobbs P, Attwell D (2006) Bidirectional control of CNS capillary diameter by pericytes. Nature 443:700–704
Hein TW, Xu W, Kuo L (2006) Dilation of retinal arterioles in response to lactate: role of nitric oxide, guanylyl cyclase, and ATP-sensitive potassium channels. Invest Ophthalmol Vis Sci 47:693–699
Yamanishi S, Katsumura K, Kobayashi T, Puro DG (2006) Extracellular lactate as a dynamic vasoactive signal in the rat retinal microvasculature. Am J Physiol Heart Circ Physiol 290:H925–H934
Yu DY, Cringle SJ, Balaratnasingam C, Morgan WH, Yu PK, Su EN (2013) Retinal ganglion cells: energetics, compartmentation, axonal transport, cytoskeletons and vulnerability. Prog Retin Eye Res 36:217–246
Acknowledgments
This work has been supported by grants from the University of Oslo, Anders Jahre’s Foundation for the Advancement of Science, and The Norwegian Research Council (including Unikard, a joint Research Council—Health Authority Grant), Norway, and from the University of Copenhagen, Velux Foundation, and the Lundbeck Foundation, Denmark.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Special Issue: In honor of Dr. Gerald Dienel.
Miriam Kolko and Fia Vosborg have contributed equally to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kolko, M., Vosborg, F., Henriksen, U.L. et al. Lactate Transport and Receptor Actions in Retina: Potential Roles in Retinal Function and Disease. Neurochem Res 41, 1229–1236 (2016). https://doi.org/10.1007/s11064-015-1792-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1792-x