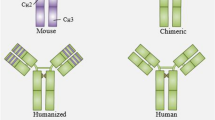
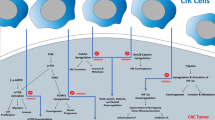
Abstract
Colorectal cancer (CRC) is the second most common cancer globally and one of the deadliest human malignancies. Traditional therapies, such as surgery, chemotherapy, and combination therapies have been used to treat patients with CRC. However, recently immunotherapy has been considered a practical and attractive therapeutic approach in various cancers, such as CRC. Among the immunotherapy methods, chimeric antigen receptor (CAR)-T, and CAR-natural killer cells (NK) cells therapy have been significantly successful, mainly in treating hematological malignancies. However, the effectiveness of CAR-T/NK cell therapy in the treatment of solid tumors, such as CRC has been less than blood malignancies due to various challenges, such as the selection of tumor antigens, lack of proper trafficking in tumor tissue, immunosuppressive tumor microenvironment, tumor heterogeneity and, adverse effects during and after CAR-T/NK cell therapy. This review summarized the biological structure of CAR-T/NK cells and their use in various types of human malignancies, particularly CRC, as well as the challenges of this type of treatment and the outcome of related combination therapies.


Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Saha S, Bardelli A, Buckhaults P, Velculescu VE, Rago C, Croix BS, Romans KE, Choti MA, Lengauer C, Kinzler KW (2001) A phosphatase associated with metastasis of colorectal cancer. Science 294:1343–1346
Markowitz SD, Bertagnolli MM (2009) Molecular basis of colorectal cancer. N Engl J Med 361:2449–2460
Fakih MG (2015) Metastatic colorectal cancer: current state and future directions. J Clin Oncol 33:1809–1824
Granados-Romero JJ, Valderrama-Treviño AI, Contreras-Flores EH, Barrera-Mera B, Herrera Enríquez M, Uriarte-Ruíz K, Ceballos-Villalba J, Estrada-Mata AG, Alvarado Rodríguez C, Arauz-Peña G (2017) Colorectal cancer: a review. Int J Res Med Sci 5:4667–4676
Silva J, Histace A, Romain O, Dray X, Granado B (2014) Toward embedded detection of polyps in wce images for early diagnosis of colorectal cancer. Int J Comput Assist Radiol Surg 9:283–293
Jass JR (2004) Hyperplastic polyps and colorectal cancer: is there a link? Clin Gastroenterol Hepatol 2:1–8
Read TE, Kodner IJ (1999) Colorectal cancer: risk factors and recommendations for early detection. Am Fam Physician 59:3083
Johnson CM, Wei C, Ensor JE, Smolenski DJ, Amos CI, Levin B, Berry DA (2013) Meta-analyses of colorectal cancer risk factors. Cancer Causes Control 24:1207–1222
Rawla P, Sunkara T, Barsouk A (2019) Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Przeglad Gastroenterologiczny 14:89
Van der Jeught K, Xu H-C, Li Y-J, Lu X-B, Ji G (2018) Drug resistance and new therapies in colorectal cancer. World J Gastroenterol 24:3834
Koido S, Ohkusa T, Homma S, Namiki Y, Takakura K, Saito K, Ito Z, Kobayashi H, Kajihara M, Uchiyama K (2013) Immunotherapy for colorectal cancer. World J Gastroenterol: WJG 19:8531
Meyerhardt JA, Mayer RJ (2005) Systemic therapy for colorectal cancer. N Engl J Med 352:476–487
Gill S, Maus MV, Porter DL (2016) Chimeric antigen receptor T cell therapy: 25 years in the making. Blood Rev 30:157–167
Wang Z, Wu Z, Liu Y, Han W (2017) New development in CAR-T cell therapy. J Hematol Oncol 10:1–11
Shin MH, Kim J, Lim SA, Kim J, Kim S-J, Lee K-M (2020) NK cell-based immunotherapies in cancer. Immune Netw. https://doi.org/10.4110/in.2020.20.e14
Newick K, O’Brien S, Moon E, Albelda SM (2017) CAR T cell therapy for solid tumors. Annu Rev Med 68:139–152
Zhao Z, Chen Y, Francisco NM, Zhang Y, Wu M (2018) The application of CAR-T cell therapy in hematological malignancies: advantages and challenges. Acta Pharmaceutica Sinica B 8:539–551
Sivori S, Meazza R, Quintarelli C, Carlomagno S, Della Chiesa M, Falco M, Moretta L, Locatelli F, Pende D (2019) NK cell-based immunotherapy for hematological malignancies. J Clin Med 8:1702
Karadimitris A (2020) Cord blood CAR-NK cells: favorable initial efficacy and toxicity but durability of clinical responses not yet clear. Cancer Cell 37:426–427
Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ (2016) Toxicity and management in CAR T-cell therapy. Mol Ther-Oncolytics 3:16011
Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F (2002) Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295:2097–2100
Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ (2005) Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105:3051–3057
Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui C-H, Leung W (2010) NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol 28:955
Hege KM, Bergsland EK, Fisher GA, Nemunaitis JJ, Warren RS, McArthur JG, Lin AA, Schlom J, June CH, Sherwin SA (2017) Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer. J Immunother Cancer 5:1–14
Xiao L, Cen D, Gan H, Sun Y, Huang N, Xiong H, Jin Q, Su L, Liu X, Wang K (2019) Adoptive transfer of NKG2D CAR mRNA-engineered natural killer cells in colorectal cancer patients. Mol Ther 27:1114–1125
Zabel M, Tauber PA, Pickl WF (2019) The making and function of CAR cells. Immunol Lett 212:53–69
Quintás-Cardama A (2019) What CAR will win the CD19 race? Mol Cancer Ther 18:498–506
Allison JP, Lanier LL (1987) Structure, function, and serology of the T-cell antigen receptor complex. Annu Rev Immunol 5:503–540
June CH, Sadelain M (2018) Chimeric antigen receptor therapy. N Engl J Med 379:64–73
Levine BL, Miskin J, Wonnacott K, Keir C (2017) Global manufacturing of CAR T cell therapy. Mol Ther- Methods Clin Dev 4:92–101
Turtle CJ, Hanafi L-A, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM (2016) CD19 CAR–T cells of defined CD4+: CD8+ composition in adult B cell all patients. J Clin Investig 126:2123–2138
Brudno JN, Kochenderfer JN (2018) Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol 15:31
Zhang C, Liu J, Zhong JF, Zhang X (2017) Engineering car-t cells. Biomarker Res 5:1–6
Siddiqi HF, Staser KW, Nambudiri VE (2018) Research techniques made simple: CAR T-cell therapy. J Investig Dermatol 138(2501–2504):e1
Kim DW, Cho J-Y (2020) Recent advances in allogeneic CAR-T cells. Biomolecules 10:263
Watanabe K, Kuramitsu S, Posey AD Jr, June CH (2018) Expanding the therapeutic window for CAR T cell therapy in solid tumors: the knowns and unknowns of CAR T cell biology. Front Immunol 9:2486
Sterner RC, Sterner RM (2021) CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J 11:1–11
Yeku OO, Brentjens RJ (2016) Armored CAR T-cells: utilizing cytokines and pro-inflammatory ligands to enhance CAR T-cell anti-tumour efficacy. Biochem Soc Trans 44:412–418
Zhao L, Cao YJ (2019) Engineered T cell therapy for cancer in the clinic. Front Immunol 10:2250
Cooper ML, Choi J, Staser K, Ritchey JK, Devenport JM, Eckardt K, Rettig MP, Wang B, Eissenberg LG, Ghobadi A (2018) An “off-the-shelf” fratricide-resistant CAR-T for the treatment of T cell hematologic malignancies. Leukemia 32:1970–1983
Fang F, Xiao W, Tian Z (2017) NK cell-based immunotherapy for cancer. Seminars in immunology. Elsevier, Amsterdam, pp 37–54
Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J (2020) CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine 59:102975
Gong Y, Wolterink RGK, Wang J, Bos GM, Germeraad WT (2021) Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol 14:1–35
Zheng L, Ren L, Kouhi A, Khawli LA, Hu P, Kaslow HR, Epstein AL (2020) A humanized Lym-1 CAR with novel DAP10/DAP12 signaling domains demonstrates reduced tonic signaling and increased antitumor activity in B-cell lymphoma models. Clin Cancer Res 26:3694–3706
Kotanides H, Sattler RM, Lebron MB, Carpenito C, Shen J, Li J, Surguladze D, Haidar JN, Burns C, Shen L (2020) Characterization of 7A5: a human CD137 (4–1BB) receptor binding monoclonal antibody with differential agonist properties that promotes antitumor immunity. Mol Cancer Ther 19:988–998
Imai C, Iwamoto S, Campana D (2005) Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 106:376–383
Chu J, Deng Y, Benson DM, He S, Hughes T, Zhang J, Peng Y, Mao H, Yi L, Ghoshal K (2014) CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia 28:917–927
Xu Y, Liu Q, Zhong M, Wang Z, Chen Z, Zhang Y, Xing H, Tian Z, Tang K, Liao X (2019) 2B4 costimulatory domain enhancing cytotoxic ability of anti-CD5 chimeric antigen receptor engineered natural killer cells against T cell malignancies. J Hematol Oncol 12:1–13
Töpfer K, Cartellieri M, Michen S, Wiedemuth R, Müller N, Lindemann D, Bachmann M, Füssel M, Schackert G, Temme A (2015) DAP12-based activating chimeric antigen receptor for NK cell tumor immunotherapy. J Immunol 194:3201–3212
Li Y, Hermanson DL, Moriarity BS, Kaufman DS (2018) Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell 23(181–192):e5
Murad JP, Kozlowska AK, Lee HJ, Ramamurthy M, Chang W-C, Yazaki P, Colcher D, Shively J, Cristea M, Forman SJ (2018) Effective targeting of TAG72+ peritoneal ovarian tumors via regional delivery of CAR-engineered T cells. Front Immunol 9:2268
Zhou R, Yazdanifar M, Roy LD, Whilding LM, Gavrill A, Maher J, Mukherjee P (2019) CAR T cells targeting the tumor MUC1 glycoprotein reduce triple-negative breast cancer growth. Front Immunol 10:1149
Aghili M, Lashkari M, Farrokhpey AH, Izadi S (2013) Triple-negative breast cancer survival in Iranian patients. Acta Med Iran 51(8):560–566
Zuo B-L, Yan B, Zheng G-X, Xi W-J, Zhang X, Yang A-G, Jia L-T (2018) Targeting and suppression of HER3-positive breast cancer by T lymphocytes expressing a heregulin chimeric antigen receptor. Cancer Immunol Immunother 67:393–401
Hillerdal V, Essand M (2015) Chimeric antigen receptor-engineered T cells for the treatment of metastatic prostate cancer. BioDrugs 29:75–89
Weimin S, Abula A, Qianghong D, Wenguang W (2020) Chimeric cytokine receptor enhancing PSMA-CAR-T cell-mediated prostate cancer regression. Cancer Biol Ther 21:570–580
Junghans RP, Ma Q, Rathore R, Gomes EM, Bais AJ, Lo AS, Abedi M, Davies RA, Cabral HJ, Al-Homsi AS (2016) Phase I trial of anti-PSMA designer CAR-T cells in prostate cancer: possible role for interacting interleukin 2-T cell pharmacodynamics as a determinant of clinical response. Prostate 76:1257–1270
Slovin SF, Wang X, Hullings M, Arauz G, Bartido S, Lewis JS, Schöder H, Zanzonico P, Scher HI, Riviere I (2013) Chimeric antigen receptor (CAR+) modified T cells targeting prostate specific membrane antigen (PSMA) in patients (pts) with castrate metastatic prostate cancer (CMPC). Am Soc Clin Oncol. https://doi.org/10.1200/jco.2013.31.6_suppl.72
Lamers CH, Sleijfer S, Van Steenbergen S, Van Elzakker P, Van Krimpen B, Groot C, Vulto A, Den Bakker M, Oosterwijk E, Debets R (2013) Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther 21:904–912
Lamers CH, Klaver Y, Gratama JW, Sleijfer S, Debets R (2016) Treatment of metastatic renal cell carcinoma (mRCC) with CAIX CAR-engineered T-cells–a completed study overview. Biochem Soc Trans 44:951–959
Jiang H, Shi Z, Wang P, Wang C, Yang L, Du G, Zhang H, Shi B, Jia J, Li Q (2019) Claudin18. 2-specific chimeric antigen receptor engineered T cells for the treatment of gastric cancer. J Natl Cancer Inst 111:409–418
Tao K, He M, Tao F, Xu G, Ye M, Zheng Y, Li Y (2018) Development of NKG2D-based chimeric antigen receptor-T cells for gastric cancer treatment. Cancer Chemother Pharmacol 82:815–827
Song Y, Tong C, Wang Y, Gao Y, Dai H, Guo Y, Zhao X, Wang Y, Wang Z, Han W (2018) Effective and persistent antitumor activity of HER2-directed CAR-T cells against gastric cancer cells in vitro and xenotransplanted tumors in vivo. Protein Cell 9:867–878
Jung M, Yang Y, McCloskey JE, Zaman M, Vedvyas Y, Zhang X, Stefanova D, Gray KD, Min IM, Zarnegar R (2020) Chimeric antigen receptor T cell therapy targeting ICAM-1 in gastric cancer. Molecular Therapy-Oncolytics 18:587–601
Koivisto L, Bi J, Häkkinen L, Larjava H (2018) Integrin αvβ6: structure, function and role in health and disease. Int J Biochem Cell Biol 99:186–196
Whilding LM, Halim L, Draper B, Parente-Pereira AC, Zabinski T, Davies DM, Maher J (2019) CAR T-cells targeting the integrin αvβ6 and coexpressing the chemokine receptor CXCR2 demonstrate enhanced homing and efficacy against several solid malignancies. Cancers 11:674
Chen X, Amar N, Zhu Y, Wang C, Xia C, Yang X, Wu D, Feng M (2020) Combined DLL3-targeted bispecific antibody with PD-1 inhibition is efficient to suppress small cell lung cancer growth. J Immunother Cancer 8:1
Wu Y, Liu H, Ding H (2016) GPC-3 in hepatocellular carcinoma: current perspectives. J Hepatocell Carcinoma 3:63
Batra SA, Rathi P, Guo L, Courtney AN, Fleurence J, Balzeau J, Shaik RS, Nguyen TP, Wu M-F, Bulsara S (2020) Glypican-3–specific CAR T cells coexpressing IL15 and IL21 have superior expansion and antitumor activity against hepatocellular carcinoma. Cancer Immunol Res 8:309–320
Smith M, Dai A, Ghilardi G, Amelsberg KV, Devlin SM, Pajarillo R, Slingerland JB, Beghi S, Herrera PS, Giardina P (2022) Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat Med 28:713–723
Chambers AM, Lupo KB, Matosevic S (2018) Tumor microenvironment-induced immunometabolic reprogramming of natural killer cells. Front Immunol 9:2517
Romanski A, Uherek C, Bug G, Seifried E, Klingemann H, Wels WS, Ottmann OG, Tonn T (2016) CD 19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J Cell Mol Med 20:1287–1294
Oelsner S, Friede ME, Zhang C, Wagner J, Badura S, Bader P, Ullrich E, Ottmann OG, Klingemann H, Tonn T (2017) Continuously expanding CAR NK-92 cells display selective cytotoxicity against B-cell leukemia and lymphoma. Cytotherapy 19:235–249
Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, Nassif Kerbauy L, Overman B, Thall P, Kaplan M (2020) Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med 382:545–553
Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu T, Yin J, You F, Zhu M, Shen W (2018) First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res 8:1083
Gang M, Marin ND, Wong P, Neal CC, Marsala L, Foster M, Schappe T, Meng W, Tran J, Schaettler M (2020) CAR-modified memory-like NK cells exhibit potent responses to NK-resistant lymphomas. Blood 136:2308–2318
You F, Wang Y, Jiang L, Zhu X, Chen D, Yuan L, An G, Meng H, Yang L (2019) A novel CD7 chimeric antigen receptor-modified NK-92MI cell line targeting T-cell acute lymphoblastic leukemia. Am J Cancer Res 9:64
Hu Z (2020) Tissue factor as a new target for CAR-NK cell immunotherapy of triple-negative breast cancer. Sci Rep 10:1–13
Murakami T, Nakazawa T, Natsume A, Nishimura F, Nakamura M, Matsuda R, Omoto K, Tanaka Y, Shida Y, Park Y-S (2018) Novel human NK cell line carrying CAR targeting EGFRvIII induces antitumor effects in glioblastoma cells. Anticancer Res 38:5049–5056
Yu M, Luo H, Fan M, Wu X, Shi B, Di S, Liu Y, Pan Z, Jiang H, Li Z (2018) Development of GPC3-specific chimeric antigen receptor-engineered natural killer cells for the treatment of hepatocellular carcinoma. Mol Ther 26:366–378
Huang Y, Zeng J, Liu T, Xu Q, Song X, Zeng J (2020) DNAM1 and 2B4 costimulatory domains enhance the cytotoxicity of anti-GPC3 chimeric antigen receptor-modified natural killer cells against hepatocellular cancer cells in vitro. Cancer Manag Res 12:3247
Huang X, Park H, Greene J, Pao J, Mulvey E, Zhou SX, Albert CM, Moy F, Sachdev D, Yee D (2015) IGF1R-and ROR1-specific CAR T cells as a potential therapy for high risk sarcomas. PLoS ONE 10:e0133152
Park H, Awasthi A, Ayello J, Chu Y, Riddell S, Rosenblum J, Lee DA, Cairo MS (2017) ROR1-specific chimeric antigen receptor (CAR) NK cell immunotherapy for high risk neuroblastomas and sarcomas. Biol Blood Marrow Transpl 23:S136–S137
Cao B, Liu M, Wang L, Liang B, Feng Y, Chen X, Shi Y, Zhang J, Ye X, Tian Y (2020) Use of chimeric antigen receptor NK-92 cells to target mesothelin in ovarian cancer. Biochem Biophys Res Commun 524:96–102
Da Y, Liu Y, Hu Y, Liu W, Ma J, Lu N, Zhang C, Zhang C (2022) STING agonist cGAMP enhances anti-tumor activity of CAR-NK cells against pancreatic cancer. Oncoimmunology 11:2054105
Marofi F, Motavalli R, Safonov VA, Thangavelu L, Yumashev AV, Alexander M, Shomali N, Chartrand MS, Pathak Y, Jarahian M (2021) CAR T cells in solid tumors: challenges and opportunities. Stem Cell Res Ther 12:1–16
Sureban SM, Berahovich R, Zhou H, Xu S, Wu L, Ding K, May R, Qu D, Bannerman-Menson E, Golubovskaya V (2020) DCLK1 monoclonal antibody-based CAR-T cells as a novel treatment strategy against human colorectal cancers. Cancers 12:54
Nakanishi Y, Seno H, Fukuoka A, Ueo T, Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M (2013) Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet 45:98–103
Deng X, Gao F, Li N, Li Q, Zhou Y, Yang T, Cai Z, Du P, Chen F, Cai J (2019) Antitumor activity of NKG2D CAR-T cells against human colorectal cancer cells in vitro and in vivo. Am J Cancer Res 9:945
Wang Y, Chen M, Wu Z, Tong C, Dai H, Guo Y, Liu Y, Huang J, Lv H, Luo C (2018) CD133-directed CAR T cells for advanced metastasis malignancies: a phase I trial. Oncoimmunology 7:e1440169
Moore PA, Shah K, Yang Y, Alderson R, Roberts P, Long V, Liu D, Li JC, Burke S, Ciccarone V (2018) Development of MGD007, a gpA33 x CD3-bispecific DART protein for T-cell immunotherapy of metastatic colorectal cancer. Mol Cancer Ther 17:1761–1772
Kempers MJ, Kuiper RP, Ockeloen CW, Chappuis PO, Hutter P, Rahner N, Schackert HK, Steinke V, Holinski-Feder E, Morak M (2011) Risk of colorectal and endometrial cancers in EPCAM deletion-positive lynch syndrome: a cohort study. Lancet Oncol 12:49–55
Zhang B-L, Li D, Gong Y-L, Huang Y, Qin D-Y, Jiang L, Liang X, Yang X, Gou H-F, Wang Y-S (2019) Preclinical evaluation of chimeric antigen receptor–modified T cells specific to epithelial cell adhesion molecule for treating colorectal cancer. Hum Gene Ther 30:402–412
Magee MS, Abraham TS, Baybutt TR, Flickinger JC, Ridge NA, Marszalowicz GP, Prajapati P, Hersperger AR, Waldman SA, Snook AE (2018) Human GUCY2C-targeted chimeric antigen receptor (CAR)-expressing T cells eliminate colorectal cancer metastases. Cancer Immunol Res 6:509–516
Baybutt T, Snook A, Waldman S, Stem J, Caparosa E, Zalewski A (2020) 105 A third-generation human GUCY2C-targeted CAR-T cell for colorectal cancer immunotherapy. BMJ Specialist J. https://doi.org/10.1136/jitc-2020-SITC2020.0105
Xiao L, Li S, Pu C, Cao Z, Yang X, Li N, Luo Y, Zhao H, Yang H, Huang X (2020) Novel CoupledCARTM technology for treating colorectal cancer. Blood 136:54
Xiao L, Li S, Pu C, Cao Z, Lu C, Hang Y, Huang X, Shen X, Wang X, Wu Z (2020) Novel CoupledCAR technology for treating colorectal cancer. Am Soc Clin Oncol. https://doi.org/10.1200/JCO.2020.38.15_suppl.e15035
Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan D-AN, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM (2011) T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 19:620–626
Zhang C, Wang Z, Yang Z, Wang M, Li S, Li Y, Zhang R, Xiong Z, Wei Z, Shen J (2017) Phase I escalating-dose trial of CAR-T therapy targeting CEA+ metastatic colorectal cancers. Mol Ther 25:1248–1258
Blat D, Zigmond E, Alteber Z, Waks T, Eshhar Z (2014) Suppression of murine colitis and its associated cancer by carcinoembryonic antigen-specific regulatory T cells. Mol Ther 22:1018–1028
Carmon KS, Lin Q, Gong X, Thomas A, Liu Q (2012) LGR5 interacts and cointernalizes with Wnt receptors to modulate Wnt/β-catenin signaling. Mol Cell Biol 32:2054–2064
McPeake DJ, Tyllis TS, Foeng J, Bandara V, Abbott CA, Gundsambuu B, Rohani-Rad E, Napoli S, Sadlon T, Barry SC (2022) In vivo characterization of a novel CAR-T cell therapy directed towards LGR5 for the treatment of colorectal cancer. Can Res 82:5574–5574
Peng X, Chen L, Chen L, Wang B, Wang Y, Zhan X (2021) Chimeric antigen receptor-natural killer cells: novel insight into immunotherapy for solid tumors. Exp Ther Med 21:1–1
Ng YY, Tay JC, Wang S (2020) CXCR1 expression to improve anti-cancer efficacy of intravenously injected CAR-NK cells in mice with peritoneal xenografts. Molecular Therapy-Oncolytics 16:75–85
Schürch CM (2020) CARving up colorectal cancer organoids in vitro. Nature Publishing Group, Berlin
Gan HK, Cvrljevic AN, Johns TG (2013) The epidermal growth factor receptor variant III (EGFRvIII): where wild things are altered. FEBS J 280:5350–5370
Giannakis M, Hodis E, Mu XJ, Yamauchi M, Rosenbluh J, Cibulskis K, Saksena G, Lawrence MS, Qian ZR, Nishihara R (2014) RNF43 is frequently mutated in colorectal and endometrial cancers. Nat Genet 46:1264–1266
Schnalzger TE, de Groot MH, Zhang C, Mosa MH, Michels BE, Röder J, Darvishi T, Wels WS, Farin HF (2019) 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J 38:e100928
D’Aloia MM, Zizzari IG, Sacchetti B, Pierelli L, Alimandi M (2018) CAR-T cells: the long and winding road to solid tumors. Cell Death Dis 9:1–12
Huang Q, Xia J, Wang L, Wang X, Ma X, Deng Q, Lu Y, Kumar M, Zhou Z, Li L (2018) miR-153 suppresses IDO1 expression and enhances CAR T cell immunotherapy. J Hematol Oncol 11:1–12
Zhang Y, Xu J, Zhang N, Chen M, Wang H, Zhu D (2019) Targeting the tumour immune microenvironment for cancer therapy in human gastrointestinal malignancies. Cancer Lett 458:123–135
Bollard CM, Rössig C, Calonge MJ, Huls MH, Wagner H-J, Massague J, Brenner MK, Heslop HE, Rooney CM (2002) Adapting a transforming growth factor β–related tumor protection strategy to enhance antitumor immunity. Blood 99:3179–3187
Forster S, Radpour R (2020) Molecular immunotherapy: promising approach to treat metastatic colorectal cancer by targeting resistant cancer cells or cancer stem cells. Front Oncol 10:2393
Feng M, Zhao Z, Yang M, Ji J, Zhu D (2021) T-cell-based immunotherapy in colorectal cancer. Cancer Lett 498:201–209
Hombach AA, Rappl G, Abken H (2019) Blocking CD30 on T cells by a dual specific CAR for CD30 and colon cancer antigens improves the CAR T cell response against CD30− tumors. Mol Ther 27:1825–1835
Sur D, Havasi A, Cainap C, Samasca G, Burz C, Balacescu O, Lupan I, Deleanu D, Irimie A (2020) Chimeric antigen receptor T-cell therapy for colorectal cancer. J Clin Med 9:182
Fedorov VD, Sadelain M, Kloss CC (2014) Novel approaches to enhance the specificity and safety of engineered T cells. Cancer J 20:160
Lee YG, Marks I, Srinivasarao M, Kanduluru AK, Mahalingam SM, Liu X, Chu H, Low PS (2019) Use of a single CAR T cell and several bispecific adapters facilitates eradication of multiple antigenically different solid tumors. Can Res 79:387–396
Zhang H, Ye Z-l, Yuan Z-g, Z-q L, Jin H-j (2016) New strategies for the treatment of solid tumors with CAR-T cells. Int J Biol Sci 12:718
Katz SC, Hardaway J, Prince E, Guha P, Cunetta M, Moody A, Wang LJ, Armenio V, Espat NJ, Junghans RP (2020) HITM-SIR: phase Ib trial of intraarterial chimeric antigen receptor T-cell therapy and selective internal radiation therapy for CEA+ liver metastases. Cancer Gene Ther 27:341–355
Xu J, Wang Y, Shi J, Liu J, Li Q, Chen L (2018) Combination therapy: a feasibility strategy for CAR-T cell therapy in the treatment of solid tumors. Oncol Lett 16:2063–2070
Hombach AA, Geumann U, Günther C, Hermann FG, Abken H (2020) IL7-IL12 engineered mesenchymal stem cells (MSCs) improve a CAR T cell attack against colorectal cancer cells. Cells 9:873
Chi X, Yang P, Zhang E, Gu J, Xu H, Li M, Gao X, Li X, Zhang Y, Xu H (2019) Significantly increased anti-tumor activity of carcinoembryonic antigen-specific chimeric antigen receptor T cells in combination with recombinant human IL-12. Cancer Med 8:4753–4765
Boland PM, Ma WW (2017) Immunotherapy for colorectal cancer. Cancers 9:50
Wang J, Lupo KB, Chambers AM, Matosevic S (2018) Purinergic targeting enhances immunotherapy of CD73+ solid tumors with piggyBac-engineered chimeric antigen receptor natural killer cells. J Immunother Cancer 6:1–14
Li G, Wu X, Chan IH, Trager JB (2020) A combination of CAR-NK and CAR-T cells results in rapid and persistent anti-tumor efficacy while reducing CAR-T cell mediated cytokine release and T-cell proliferation. Am Assoc Cancer Res. https://doi.org/10.1158/1538-7445.AM2020-4235
Zhang Q, Zhang H, Ding J, Liu H, Li H, Li H, Lu M, Miao Y, Li L, Zheng J (2018) Combination therapy with EpCAM-CAR-NK-92 cells and regorafenib against human colorectal cancer models. J Immunol Res. https://doi.org/10.1155/2018/4263520
Acknowledgements
None
Funding
None.
Author information
Authors and Affiliations
Contributions
WP: Conception, design and inviting co-authors to participate. HW: Writing original manuscript draft. WP: Review and editing of manuscript critically for important intellectual content and provided comments and feedback for the scientific contents of the manuscript. All authors read, revised and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Pan, W. Challenges of chimeric antigen receptor-T/natural killer cell therapy in the treatment of solid tumors: focus on colorectal cancer and evaluation of combination therapies. Mol Cell Biochem 478, 967–980 (2023). https://doi.org/10.1007/s11010-022-04568-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04568-0