Abstract
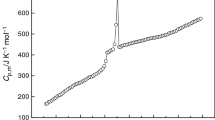
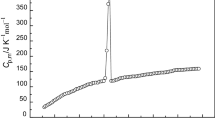
The molar heat capacities of the room temperature ionic liquid 1-butylpyridinium tetrafluoroborate (BPBF4) were measured by an adiabatic calorimeter in temperature range from 80 to 390 K. The dependence of the molar heat capacity on temperature is given as a function of the reduced temperature X by polynomial equations, C p,m [J K−1 mol−1]=181.43+51.297X −4.7816X 2−1.9734X 3+8.1048X 4+11.108X 5 [X=(T−135)/55] for the solid phase (80–190 K), C p,m [J K−1 mol−1]= 349.96+25.106X+9.1320X 2+19.368X 3+2.23X 4−8.8201X 5 [X=(T−225)/27] for the glass state (198–252 K), and C p,m[J K−1 mol−1]= 402.40+21.982X−3.0304X 2+3.6514X 3+3.4585X 4 [X=(T−338)/52] for the liquid phase (286–390 K), respectively. According to the polynomial equations and thermodynamic relationship, the values of thermodynamic function of the BPBF4 relative to 298.15 K were calculated in temperature range from 80 to 390 K with an interval of 5 K. The glass transition of BPBF4 was observed at 194.09 K, the enthalpy and entropy of the glass transition were determined to be ΔH g=2.157 kJ mol−1 and ΔS g=11.12 J K−1 mol−1, respectively. The result showed that the melting point of the BPBF4 is 279.79 K, the enthalpy and entropy of phase transition were calculated to be ΔH m = 8.453 kJ mol−1 and ΔS m=30.21 J K−1 mol−1. Using oxygen-bomb combustion calorimeter, the molar enthalpy of combustion of BPBF4 was determined to be Δc H 0m = −5451±3 kJ mol−1. The standard molar enthalpy of formation of BPBF4 was evaluated to be Δf H 0m = −1356.3±0.8 kJ mol−1 at T=298.150±0.001 K.
Similar content being viewed by others
References
C. M. Gordon, J. D. Holbrey, A. R. Kennedy and K. R. Seddon, J. Mater. Chem., 8 (1998) 2627.
J. Fuller, R. T. Cartin and R. A. Osteryoung, J. Electrochem. Soc., 144 (1997) 3881.
J. Sun, M. Forsyth and D. R. Macfarlane, J. Phys. Chem. B, 102 (1998) 8858.
T. Welton, Chem. Rev., 99 (1999) 2071.
A. J. Carmichael and K. R. Seddon, J. Phys. Org. Chem., 13 (2000) 591.
C. E. Song, W. H. Shim, E. J. Roh, S. G. Lee and J. H. Choi, Chem. Commun., 12 (2001) 1122.
P. Wasserscheid, C. M. Gordon, C. Hilgers, M. J. Muldoon and I. R. Dunkin, Chem. Commun., 13 (2001) 1186.
C. Wheeler, K. N. West, C. L. Liotta and C. A. Eckert, Chem. Commun., 10 (2001) 887.
F. Endres, Phys. Chem. Chem. Phys., 3 (2001) 3165.
V. Najdanovic-Visak, J. M. S. S. Esperanca and L. P. N. Rebelo, Phys. Chem. Chem. Phys., 4 (2002) 1701.
P. Vasserscheid and W. Keim, Angew. Chem. Int. Ed., 39 (2000) 3772.
J. D. Holbrey and K. R. Seddon, Clean Products Processes, 1 (1999) 223.
D. Appleby, C. L. Hussey, K. R. Seddon and J. E. Turp, Nature, 323 (1986) 614.
J. L. Anthony, F. J. Maginn and Brennecke, J. Phys. Chem. B, 105 (2001) 10942.
J. Z. Yang, P. Tian, L. L. He and W. G. Xu, Fluid Phase Equilib., 204 (2003) 295.
J. Z. Yang, W. G. Xu and Q. G. Zhang, J. Chem. Thermodyn., 35 (2003) 1855.
J. Z. Yang, P. Tian and W. G. Xu, Thermochim. Acta, 412 (2004) 1.
J. D. Holbrey, W. M. Reichert and R. P. Swatloski, Green Chem., 4 (2002) 407.
J. Fuller, R. A. Osteryoung and R. T. Carlin, J. Electrochem. Soc., 142 (1995) 3632.
J. Fuller, R. T. Carlin and R. A. Osteryoung, J. Electrochem. Soc., 144 (1997) 3881.
E. N. Jacobsen, I. Marko and K. B. Sharpless, J. Am. Chem. Soc., 110 (1988) 1986.
P. A. Z. Suarez, J. E. L. Dullius, S. Einloft, R. F. D. Souza and J. Dupnot, Polyhedron, 157 (1996) 1217.
P. J. Dyson, M. C. Grossel, N. Srinivasan, T. Vine, T. Welton, D. J. Williams, A. J. P. White and T. Zigras, DALTON, 19 (1997) 3465.
A. Noda and M. Watanabe, Electrochim. Acta, 45 (2000) 1265.
J. Robinson and R. A. Osteryoung, J. Am. Chem. Soc., 102 (1979) 323.
W. D. Bancroft and S. S. Hubard, J. Am. Chem. Soc., 64 (1942) 347.
Z. C. Tan, G. Y. Sun, Y. Sun, A. X. Yin, W. B. Wang, J. C. Ye and L. X. Zhou, J. Thermal Anal., 45 (1995) 59.
D. G. Archer, J. Phys. Chem. Ref. Data, 22 (1993) 1441.
R. L. David, CRC Handbook of Chemistry and Physics, 80th Ed., CRC Press, Boca Raton 1999, Chapter 6-6.
K. K. Kelley, J. Am. Chem. Soc., 51 (1929) 779.
H. M. Huffman, G. S. Parks and A. C. Daniels, J. Am. Chem. Soc., 52 (1930) 1547.
H. Osamu, S. Hiroshi and S. Syuzo, J. Chem. Thermodyn., 9 (1977) 1133.
Y. Y. Di, Z. C. Tan, X. H. Sun, M. H. Wang, F. Xu, Y. F. Liu, L. X. Sun and H. T. Zhang, J. Chem. Thermodyn., 36 (2004) 79.
S. X. Wang, Z. C. Tan, Y. Y. Di, F. Xu, M. H. Wang, L. X. Sun and T. Zhang, J. Therm. Anal. Cal., 76 (2004) 335.
F. Xu, L. X. Sun, Z. C. Tan, J. G. Liang, Y. Y. Di, Q. F. Tian and T. Zhang, J. Therm. Anal. Cal., 76 (2004) 481.
Z. D. Nan and Z. C. Tan, J. Therm. Anal. Cal., 76 (2004) 955.
B. Xue, J. Y. Wang, Z. C. Tan, S. W. Lu and S. H. Meng, J. Therm. Anal. Cal., 76 (2004) 965.
S. Tomitaka, M. Mizukami, F. Paladi and M. Oguni, J. Therm. Anal. Cal., 81 (2005) 637.
Z. C. Tan, B. Xue, S. W. Lu, S. H. Meng, X. H. Yuan and Y. J. Song, J. Therm. Anal. Cal., 63 (2001) 297.
H. A. Skinner, Experimental Thermochemistry, 2 (1962) 19.
J. D. Cox, D. D. Wagman and V. A. Medvedev, CODATA Key Values for Thermodynamics. Hemisphere, New York 1989.
J. D. Cox, J. Chem. Thermodyn., 10 (1978) 903.
M. W. Chase Jr., NIST-JANAF Themochemical Tables, Fourth Edition, J. Phys. Chem. Ref. Data, Monograph, 9 (1998) 1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Z.H., Sun, L.X., Tan, Z.C. et al. Thermodynamic investigation of room temperature ionic liquid. J Therm Anal Calorim 89, 289–294 (2007). https://doi.org/10.1007/s10973-006-7511-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-006-7511-8