Abstract
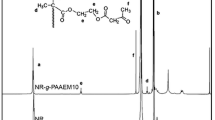
The concept of using glutaraldehyde (GTA) to crosslink natural rubber (NR) particles bearing diacetone acrylamide (DAAM) functional groups during film formation was investigated in the present work. The primary advantage of this curing system is that it is feasible under ambient conditions, which can lower operating costs of the curing process. Graft copolymers of NR and poly(diacetone acrylamide) prepared with 5 wt% of DAAM (NR–g–PDAAM5) were synthesized by seeded emulsion polymerization at 50 °C. Then, the tensile properties were measured for cast films formed from NR–g–PDAAM5 latex in the absence and presence of GTA. The results revealed increased tensile strength of the NR–g–PDAAM5 film, when GTA was added into the latex prior to film casting. The crosslinking of NR–g–PDAAM5 latex film by reaction with GTA, after film casting, was also investigated using attenuated total reflection Fourier transform infrared (ATR–FTIR) and dynamic mechanical thermal analysis (DMTA). ATR–FTIR analysis demonstrated that crosslinking reactions formed conjugated C=C double bonds between the active carbonyl groups of DAAM and GTA. The complementary use of DMTA also corroborated that crosslinking reactions took place involving the grafted PDAAM chains on the NR particles. This was evidenced by a clear shift towards higher temperatures of the tan δ peak, corresponding to the Tg of NR–g–PDAAM phase, when GTA was incorporated into the NR–g–PDAAM5 latex before film formation. Additionally, a noticeable increase in thermal stability of the NR–g–PDAAM5 film was also observed with added GTA. Hence, it can be concluded that GTA is an efficient room-temperature crosslinker for NR particles functionalized with DAAM. This curing system can also be considered an alternative, simple, and inexpensive method for curing NR latex compounds, as only one component (GTA) is required in the curing process.














Similar content being viewed by others
References
Feng J, Pham H, Macdonald P, Winnik MA, Geurts JM, Zirkzee H, van Es S, German AL(1998) J Coat Technol 70:57
Kessel N, Illsley DR, Keddie JL (2008) J Coat Technol Res 3:285
Foster AB, Lovell PA, Rabjohns MA (2009) Polymer 50:654
Tale NV, Jagtap RN (2010) Iran Polym J 19:801
Zhang X, Liu Y, Huang H, Li Y, Chen H (2012) J Appl Polym Sci 123:1822
Jones FN, Nichols ME, Pappas SP (2017) Organic coatings: science and technology, 4th edn. Wiley, Hoboken
Thongnuanchan B, Ninjan R, Kaesaman A, Nakason C (2015) Polym Bull 72:135
Olde Damink LHH, Dijkstra PJ, Van Luyn MJA, Van Wachem PB, Nieuwenhuis P, Feijen J (1995) J Mater Sci Mater Med 6:460
Farris S, Song J, Huang Q (2010) J Agric Food Chem 58:998
Kiernan JA (2000) Microsc Today 1:8
Gebben B, van den Berg HWA, Bargeman D, Smolders CA (1985) Polymer 26:1737
Dai S, Barbari TA (1999) J Membr Sci 156:67
Alemzadeh I, Vossoughi M (2002) Chem Eng Process 41:707
Figueiredo KCS, Alves TLM, Borges CP (2009) J Appl Polym Sci 111:3074
Kumbar SG, Soppimath KS, Aminabhavi TM (2003) J Appl Polym Sci 87:1525
Dmitriev I, Kuryndin I, Bobrova N, Smirnov M (2015) Mater Today Commun 4:93
Pye DJ (1960) Polymer composition and method. US Patent 2,960,486
Zweigle ML (1973) Removal of monomer from acrylamide polymers with sulfur dioxide. US Patent 3,780,006
Ellis B, Welding GN (1964) Rubber Chem Technol 37:563
Flory PJ, Rehener J (1943) J Chem Phys 11:521
Hagen R, Salmen L, Stenberg B (1996) J Polym Sci 34:1997
Gent AN, Kawahara S, Zhao J (1998) Rubber Chem Technol 71:668
Trabelsi S, Albouy P-A, Rault J (2002) Macromolecules 35:10054
Tosaka M, Kawakami D, Senoo K, Kohjiya S, Ikeda Y, Toki S, Hsiao BS (2006) Macromolecules 39:5100
Chenal J-M, Chazeau L, Guy L, Bomal Y, Gauthier C (2007) Polymer 48:1042
Huneau B (2011) Rubber Chem Technol 84:425
Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC (2004) BioTechniques 37:790
Wang Y, Mo X, Sun XS, Wang D (2007) J Appl Polym Sci 104:130
Eng AH, Ong EL (2001) In: Bhowmick AK, S Howard (ed) Handbook of elastomers, 2nd edn. Marcel Dekker, Inc., New York, pp 29–60
Johns J, Nakason C, Thitithammawong A, Klinpituksa P (2012) Rubber Chem Technol 85:565
Honeycutt T, Flowery B (2005) Decreasing allergenicity of natural latex rubber prior to vulcanization. US Patent 20050277722 A1
Honeycutt T (2006) Decreasing allergenicity of natural latex rubber prior to vulcanization. US Patent 7056970 B2
Honeycutt T, William D, Matthew C, Russell C, Mark S (2007) Rubber World 237:32
Honeycutt T, Sharivker V, Sharivker S, Blinov V, Doyle W (2005) In international latex conference papers, Charlotte
Nakason C, Kaesaman A, Yimwan N (2003) J Appl Polym Sci 87:68
Smith MB (2015) Organic chemistry: an acid–base approach, 2nd edn. CRC Press, Boca Raton
Thongnuanchan B, Ninjan R, Kaesaman A, Nakason C (2015) J Polym Res 22:115
Ebewele RO (2000) Polymer science and technology. CRC Press LLC, Boca Raton
Hutchinson JM (1997) In: Haward RN, Young RJ (eds) The physics of glassy polymers, 2nd edn. Springer, London, pp 128–138
Phillips JC (1979) J Non-Cryst Solids 34:153
George S, Neelakantan NR, Varughese KT, Thomas S (1997) J Polym Sci B 35:2309
Burrows HD, Ellis HA, Utah SI (1981) Polymer 22:1740
Ozawa T (1965) Bull Chem Soc Jpn 38:1881
Park JW, Oh SC, Lee HP, Kim HT, Yoo KO (2000) Polym Degrad Stab 67:535
Ceamanos J, Mastral JF, Millera A, Aldea ME (2002) J Anal Appl Pyrol 65:93
Popescu C (1996), Thermochim Acta 285:309
Kim W, Kim SD, Lee SB, Hong IN (2000) J Ind Eng Chem 6:348
Acknowledgements
This work was supported by the Research Fund of Prince of Songkla University, SAT581267S. The authors would like to thank the Research and Development Office (RDO) and Assoc. Prof. Seppo Karrila for editing this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thongnuanchan, B., Ninjan, R., Kalkornsurapranee, E. et al. Glutaraldehyde as Ambient Temperature Crosslinking Agent of Latex Films from Natural Rubber Grafted with Poly(diacetone acrylamide). J Polym Environ 26, 3069–3085 (2018). https://doi.org/10.1007/s10924-018-1193-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-018-1193-8