Abstract
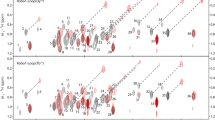
The zero- and double-quantum methyl TROSY Hahn-echo and the methyl 1H–1H dipole–dipole cross-correlation nuclear magnetic resonance experiments enable estimation of multiple quantum chemical exchange broadening in methyl groups in proteins. The two relaxation rate constants are established to be linearly dependent using molecular dynamics simulations and empirical analysis of experimental data. This relationship allows chemical exchange broadening to be recognized as an increase in the Hahn-echo relaxation rate constant. The approach is illustrated by analyzing relaxation data collected at three temperatures for E. coli ribonuclease HI and by analyzing relaxation data collected for different cofactor and substrate complexes of E. coli AlkB.





Similar content being viewed by others
References
Ban D, Mazur A, Carneiro MG, Sabo TM, Giller K, Koharudin LM, Becker S, Gronenborn AM, Griesinger C, Lee D (2013) Enhanced accuracy of kinetic information from CT-CPMG experiments by transverse rotating-frame spectroscopy. J Biomol NMR 57:73–82
Banks JL, Beard HS, Cao Y, Cho AE, Damm W, Farid R, Felts AK, Halgren TA, Mainz DT, Maple JR, Murphy R, Philipp DM, Repasky MP, Zhang LY, Berne BJ, Friesner RA, Gallicchio E, Levy RM (2005) Integrated Modeling Program, Applied Chemical Theory (IMPACT). J Comput Chem 26:1752–1780
Bleijlevens B, Shivarattan T, Flashman E, Yang Y, Simpson PJ, Koivisto P, Sedgwick B, Schofield CJ, Matthews SJ (2008) Dynamic states of the DNA repair enzyme AlkB regulate product release. EMBO Rep 9:872–877
Bleijlevens B, Shivarattan T, van den Boom KS, de Haan A, van der Zwan G, Simpson PJ, Matthews SJ (2012) Changes in protein dynamics of the DNA repair dioxygenase AlkB upon binding of Fe2+ and 2-oxoglutarate. Biochemistry 51:3334–3341
Butterwick JA, Palmer AG (2006) An inserted gly residue fine tunes dynamics between mesophilic and thermophilic ribonucleases H. Protein Sci 15:2697–2707
Butterwick JA, Loria P, Astrof J, Kroenke NS, Cole CD, Rance R, M. & Palmer AG (2004) Multiple time scale backbone dynamics of homologous thermophilic and mesophilic ribonuclease HI enzymes. J Mol Biol 339:855–871
Cheatham TE III, Miller JL, Fox T, Darden TA, Kollman PA (1995) Molecular dynamics simulations on solvated biomolecular systems: The particle mesh Ewald method leads to stable trajectories of DNA, RNA, and proteins. J Am Chem Soc 117:4193–4194
Chen VB, Arendall WB 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 66:12–21
Darden T, York D, Pedersen L (1993) Particle mesh Ewald: an N⋅log(N) method for Ewald sums in large systems. J Chem Phys 98:10089–10092
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Ergel B, Gill ML, Brown L, Yu B, Palmer AG, Hunt JF (2014) Protein dynamics control the progression and efficiency of the catalytic reaction cycle of the Escherichia coli DNA-repair enzyme AlkB. J Biol Chem 289:29584–29601
Findeisen M, Brand T, Berger S (2007) A 1H–NMR thermometer suitable for cryoprobes. Magn Reson Chem 45:175–178
Fushman D, Cowburn D (1998) Model-independent analysis of 15N chemical shift anisotropy from NMR relaxation data. Ubiquitin as a test example. J Am Chem Soc 120:7109–7110
Gill ML, Palmer AG (2011) Multiplet-filtered and gradient-selected zero-quantum TROSY experiments for 13C1H3 methyl groups in proteins. J Biomol NMR 51:245–251
Goddard T, Kneller DG (2008) SPARKY 3. University of California, San Francisco
Hansen DF, Feng H, Zhou Z, Bai Y, Kay LE (2009) Selective characterization of microsecond motions in proteins by NMR relaxation. J Am Chem Soc 131:16257–16265
Helmus JJ, Jaroniec CP (2013) NMRglue: an open source Python package for the analysis of multidimensional NMR data. J Biomol NMR 55:355–367
Hoover WG (1985) Canonical dynamics: equilibrium phase-space distributions. Phys Rev A 31:1695–1697
Hsu A, O’ Brien PA, Bhattacharya S, Rance M, Palmer AG (2018) Enhanced spectral density mapping through combined multiple-field deuterium 13CH2D methyl spin relaxation NMR spectroscopy. Methods 139:76–84
Hunter JD, Matplotlib (2007) A 2D graphics environment. IEEE Comput Sci Eng 9:90–95
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935
Konrat R, Sterk H (1993) Cross-correlation effects in the transverse relaxation of multiple-quantum transitions of heternuclear spin systems. Chem Phys Lett 203:75–80
Korzhnev DM, Kloiber K, Kay LE (2004a) Multiple-quantum relaxation dispersion NMR spectroscopy probing millisecond time-scale dynamics in proteins: theory and application. J Am Chem Soc 126:7320–7329
Korzhnev DM, Kloiber K, Kanelis V, Tugarinov V, Kay LE (2004b) Probing slow dynamics in high molecular weight proteins by methyl-TROSY NMR spectroscopy: application to a 723-residue enzyme. J Am Chem Soc 126:3964–3973
Kroenke CD, Loria JP, Lee LK, Rance M, Palmer AG (1998) Longitudinal and transverse 1H–15N dipolar/15N chemical shift anisotropy relaxation interference: unamiguous determination of rotational diffusion tensors and chemical exchange effects in biological macromolecules. J Am Chem Soc 120:7905–7915
Martyna GJ, Tobias DJ, Klein ML (1994) Constant pressure molecular dynamics algorithms. J Chem Phys 101:4177–4189
McKinney W (2010) Data structures for statistical computing in Python. In: van der Walt S, Millman J (eds) Proceedings of the 9th Python in science conference, pp 51–56
Millet O, Palmer AG (2000) The static magnetic field dependence of chemical exchange linebroadening defines the chemical shift time scale. J Am Chem Soc 122:2867–2877
Millman KJ, Aivazis M (2011) Python for scientists and engineers. IEEE Comput Sci Eng 13:9–12
Ming D, Brüschweiler R (2004) Prediction of methyl-side chain dynamics in proteins. J Biomol NMR 29:363–368
Norwood TJ, Tillett ML, Lian L-Y (1999) Influence of cross-correlation between the chemical shift anisotropies of pairs of nuclei on multiple-quantum relaxation rates in macromolecules. Chem Phys Lett 300:429–434
Nosé S (1984) A unified formulation of the constant temperature molecular dynamics methods. J Chem Phys 81:511–519
O’Connell NE, Grey MJ, Tang Y, Kosuri P, Miloushev VZ, Raleigh DP, Palmer AG (2009) Partially folded equilibrium intermediate of the villin headpiece HP67 defined by 13C relaxation dispersion. J Biomol NMR 45:85–98
Oliphant TE (2007) Python for scientific computing. IEEE Comput Sci Eng 9:10–20
Palmer AG, Koss H (2019) Chemical exchange. Methods Enzymol 615:177–236
Pérez F, Granger BE, IPython (2007) A system for interactive scientific computing. IEEE Comput Sci Eng 9:21–29
Phan IQH, Boyd J, Campbell ID (1996) Dynamic studies of a fibronectin type I module pair at three frequencies: Anisotropic modelling and direct determination of conformational exchange. J Biomol NMR 8:369–378
Reddy JG, Pratihar S, Ban D, Frischkorn S, Becker S, Griesinger C, Lee D (2018) Simultaneous determination of fast and slow dynamics in molecules using extreme CPMG relaxation dispersion experiments. J Biomol NMR 70:1–9
Sastry GM, Adzhigirey M, Annabhimoju TD, R. & Sherman W (2013) Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput Aided Mol Des 27:221–234
Stafford KA, Robustelli P, Palmer AG (2013) Thermal adaptation of conformational dynamics in ribonuclease H. PLoS Comput Biol 9:e1003218
Stafford KA, Trbovic N, Butterwick JA, Abel R, Friesner RA, Palmer AG (2015) Conformational preferences underlying reduced activity of a thermophilic ribonuclease H. J Mol Biol 427:853–866
Toyama Y, Osawa M, Yokogawa M, Shimada I (2016) NMR method for characterizing microsecond-to-millisecond chemical exchanges utilizing differential multiple-quantum relaxation in high molecular weight proteins. J Am Chem Soc 138:2302–2311
Toyama Y, Kano H, Mase Y, Yokogawa M, Osawa M, Shimada I (2017) Dynamic regulation of GDP binding to G proteins revealed by magnetic field-dependent NMR relaxation analyses. Nat Commun 8:14523
Trott O, Siggers K, Rost B, Palmer AG (2008) Protein conformational flexibility prediction using machine learning. J Magn Reson 192:37–47
Tuckerman M, Berne BJ, Martyna GJ (1992) Reversible multiple time scale molecular dynamics. J Chem Phys 97:1990–2001
Tugarinov V, Kay LE (2006) Relaxation rates of degenerate 1H transitions in methyl groups of poteins as reporters of side-chain dynamics. J Am Chem Soc 128:7299–7308
Tugarinov V, Kay LE (2007) Separating degenerate 1H transitions in methyl group probes for single-quantum 1H–CPMG relaxation dispersion NMR spectroscopy. J Am Chem Soc 129:9514–9521
Tugarinov V, Hwang PM, Ollerenshaw JE, Kay LE (2003) Cross-correlated relaxation enhanced 1H–13C NMR spectroscopy of methyl groups in very high molecular weight proteins and protein complexes. J Am Chem Soc 125:10420–10428
Tugarinov V, Sprangers R, Kay LE (2004) Line narrowing in methyl-TROSY using zero-quantum 1H–13C NMR spectroscopy. J Am Chem Soc 126:4921–4925
Tugarinov V, Kay LE, Ibraghimov I, Orekhov VY (2005) High-resolution four-dimensional 1H–13C NOE spectroscopy using methyl-TROSY, sparse data acquisition, and multidimensional decomposition. J Am Chem Soc 127:2767–2775
Tugarinov V, Ollerenshaw JE, Kay LE (2006) Dipolar dynamic frequency shifts in multiple-quantum spectra of methyl groups in proteins: correlation with side-chain motion. Magn Reson Chem 44:S122–S129
Tugarinov V, Sprangers R, Kay LE (2007) Probing side-chain dynamics in the proteasome by relaxation violated coherence transfer NMR spectroscopy. J Am Chem Soc 129:1743–1750
van der Walt S, Colbert SC, Varoquaux G (2011) The NumPy array: a structure for efficient numerical computation. IEEE Comput Sci Eng 13:22–30
Wang C, Palmer AG (2002) Differential multiple quantum relaxation caused by chemical exchange outside the fast exchange limit. J Biomol NMR 24:263–268
Wang C, Palmer AG (2003) Solution NMR methods for quantitative identification of chemical exchange in 15N-labeled proteins. Magn Reson Chem 41:866–876
Williams CJ, Headd JJ, Moriarty NW, Prisant MG, Videau LL, Deis LN, Verma V, Keedy DA, Hintze BJ, Chen VB, Jain S, Lewis SM, Arendall WB, Snoeyink J, Adams PD, Lovell SC, Richardson JS, Richardson DC, MolProbity (2018) More and better reference data for improved all-atom structure validation. Protein Sci 27:293–315
York DM, Darden TA, Pedersen LG (1993) The effect of long-range electrostatic interactions in simulations of macromolecular crystals: a comparison of the Ewald and truncated list methods. J Chem Phys 99:8345–8348
Zhang F, Brüschweiler R (2002) Contact model for the prediction of NMR N–H order parameters in globular proteins. J Am Chem Soc 124:12654–12655
Acknowledgements
Support from National Institutes of Health grants GM089047 (M.L.G.), GM008281 (A. H.). and GM050291 (A.G.P.) is acknowledged gratefully. The AVANCE 600 NMR spectrometer at Columbia University was purchased with the support of NIH grant RR026540. Some of the work presented here was conducted at the Center on Macromolecular Dynamics by NMR Spectroscopy located at the New York Structural Biology Center, supported by a grant from the NIH National Institute of General Medical Sciences (P41 GM118302). A.G.P. is a member of the New York Structural Biology Center. A preliminary account of this work was presented as poster 96 at the 56th Experimental NMR Conference (2016). We thank Richard Friesner (Columbia University) and Martha Beckwith (Advanced Science Research Center, City University of New York) for helpful discussions and Richard Friesner for access to computational facilities. This paper is dedicated to Dennis Torchia (National Institutes of Health) on occasion of his 80th birthday in appreciation of his pioneering achievements in NMR spectroscopy, spin relaxation, and protein dynamics.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gill, M.L., Hsu, A. & Palmer, A.G. Detection of chemical exchange in methyl groups of macromolecules. J Biomol NMR 73, 443–450 (2019). https://doi.org/10.1007/s10858-019-00240-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-019-00240-w