Abstract
Purpose
Polycystic ovary syndrome (PCOS) is an endocrine-metabolic disorder that leads to lower natural reproductive potential and presents a challenge for assisted reproductive medicine because patients may exhibit immature oocyte retrieval and a higher risk of ovarian hyper stimulation syndrome during in vitro fertilization (IVF) treatment. This study aimed to identify potential lipid biomarkers for women with PCOS and a hyper response to controlled ovarian stimulation.
Methods
Follicular fluid samples were collected from patients who underwent IVF, including normal responder women who became pregnant (control group, n = 11), women with PCOS and a hyper response to gonadotropins (PCOS group, n = 7) and women with only hyper response to gonadotropins (HR group, n = 7). A lipidomic analysis was performed by electrospray ionization mass spectrometry, and candidate biomarkers were analyzed by tandem mass spectrometry experiment.
Results
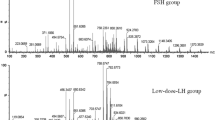
The lipid profiles indicated particularities related to differences in phosphatidylcholine (PCOS and HR), phosphatidylserine, phosphatydilinositol and phosphatidylglycerol (control), sphingolipids (PCOS) and phosphatidylethanolamine (control and HR).
Conclusions
These findings contribute to the understanding of the molecular mechanisms associated with lipid metabolism in the PCOS-related hyper response, and strongly suggest that these lipids may be useful as biomarkers, leading to the development of more individualized treatment for pregnancy outcome.



Similar content being viewed by others
References
Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;24:1223–36.
Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800.
Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41–7.
Vieira RC, Barcelos ID, Ferreira EM, de Araújo MC, dos Reis RM, Ferriani RA, et al. Evaluation of meiotic abnormalities of oocytes from policystic ovary syndrome patients submitted to ovarian stimulation. Rev Bras Ginecol Obstet. 2008;30:241–7.
MacDougall MJ, Tan SL, Balen A, Jacobs HS. A controlled study comparing patients with and without polycystic ovaries undergoing in-vitro fertilization. Hum Reprod. 1993;8:233–7.
Fortune JE. Ovarian follicular growth and development in mammals. Biol Reprod. 1994;50:225–32.
Leroy JL, Vanholder T, Delanghe JR, Opsomer G, Van Soom A, Bols PE, et al. Metabolic changes in follicular fluid of the dominant follicle in high-yielding dairy cows early post partum. Theriog. 2004;15:1131–43.
Lam SM, Shui G. Lipidomics as a principal tool for advancing biomedical research. J Genet Genomics. 2013;20:375–90.
Zehethofer N, Pinto DM. Recent developments in tandem mass spectrometry for lipidomic analysis. Anal Chim Acta. 2008;3:62–70.
Roberts LD, McCombie G, Titman CM, Griffin JL. A matter of fat: an introduction to lipidomic profiling methods. J Chromatogr B Anal Technol Biomed Life Sci. 2008;15:174–81.
Lucki NC, Sewer MB. Multiple roles for sphingolipids in steroid hormone biosynthesis. Subcell Biochem. 2008;49:387–412.
Montani DA, Cordeiro FB, Regiani T, Victorino AB, Pilau EJ, Gozzo FC, et al. The follicular microenviroment as a predictor of pregnancy: MALDI-TOF MS lipid profile in cumulus cells. J Assist Reprod Genet. 2012;29:1289–97.
Shaaker M, Rahimipour A, Nouri M, Khanaki K, Darabi M, Farzadi L, et al. Fatty acid composition of human follicular fluid phospholipids and fertilization rate in assisted reproductive techniques. Iran Biomed J. 2012;16:162–8.
Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989;6:64–71.
Iwasaki Y, Nakano Y, Mochizuki K, Nomoto M, Takahashi Y, Ito R, et al. A new strategy for ionization enhancement by derivatization for mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2011;15:1159–65.
Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7.
Nastri CO, Ferriani RA, Rocha IA, Martins WP. Ovarian hyperstimulation syndrome: pathophysiology and prevention. J Assist Reprod Genet. 2010;27:121–8.
Belosi C, Selvaggi L, Apa R, Guido M, Romualdi D, Fulghesu AM, et al. Is the PCOS diagnosis solved by ESHRE/ASRM 2003 consensus or could it include ultrasound examination of the ovarian stroma? Hum Reprod. 2006;21:3108–15.
Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, et al. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;27:1017–21.
Fauser BC, Pache TD, Lamberts SW, Hop WC, de Jong FH, Dahl KD. Serum bioactive and immunoreactive luteinizing hormone and follicle-stimulating hormone levels in women with cycle abnormalities, with or without polycystic ovarian disease. J Clin Endocrinol Metab. 1991;73:811–7.
Taylor AE, McCourt B, Martin KA, Anderson EJ, Adams JM, Schoenfeld D, et al. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:2248–56.
Catteau-Jonard S, Bancquart J, Poncelet E, Lefebvre-Maunoury C, Robin G, Dewailly D. Polycystic ovaries at ultrasound: normal variant or silent polycystic ovary syndrome? Ultrasound Obstet Gynecol. 2012;40:223–9.
Kdous M, Chaker A, Zhioua A, Zhioua F. Oocyte and embryo quality and outcome of ICSI cycles in patients with polycystic ovary syndrome (PCOS) versus normo-ovulatory. J Gynecol Obstet Biol Reprod. 2009;38:133–43.
Lykidis A. Comparative genomics and evolution of eukaryotic phospholipid biosynthesis. Prog Lipid Res. 2007;46:171–99.
Verhoven B, Schlegel RA, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995;1:1597–601.
Cui Z, Houweling M. Phosphatidylcholine and cell death. Biochim Biophys Acta. 2002;30:87–96.
Kawasaki K, Kuge O, Chang SC, Heacock PN, Rho M, Suzuki K, et al. Isolation of a chinese hamster ovary (CHO) cDNA encoding phosphatidylglycerophosphate (PGP) synthase, expression of which corrects the mitochondrial abnormalities of a PGP synthase-defective mutant of CHO-K1 cells. J Biol Chem. 1999;15:1828–34.
Cataldi T, Cordeiro FB, Costa L do V, Pilau EJ, Ferreira CR, Gozzo FC, Eberlin MN, Bertolla RP, Cedenho AP, Turco EG. Lipid profiling of follicular fluid from women undergoing IVF: young poor ovarian responders versus normal responders. Hum Fertil. 2013; 16:269–77.
Balasubramanian K, Mirnikjoo B, Schroit AJ. Regulated externalization of phosphatidylserine at the cell surface: implications for apoptosis. J Biol Chem. 2007;282:18357–64.
Schroit AJ, Zwaal RF. Transbilayer movement of phospholipids in red cell and platelet membranes. Biochim Biophys Acta. 1991;13:313–29.
Balla T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev. 2013;93:1019–137.
Croze ML, Soulage CO. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie. 2013;95:811–27.
Ciotta L, Stracquadanio M, Pagano I, Carbonaro A, Palumbo M, Gulino F. Effects of myo-inositol supplementation on oocyte’s quality in PCOS patients: a double blind trial. Eur Rev Med Pharmacol Sci. 2011;15:509–14.
Bou Khalil M, Hou W, Zhou H, Elisma F, Swayne LA, Blanchard AP, et al. Lipidomics era: accomplishments and challenges. Mass Spectrom Rev. 2010;29:877–929.
van Echten-Deckert G, Walter J. Sphingolipids: critical players in Alzheimer’s disease. Prog Lipid Res. 2012;51:378–93.
Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279:2610–23.
Straczkowski M, Kowalska I. The role of skeletal muscle sphingolipids in the development of insulin resistance. Rev Diabet Stud. 2008;5:13–24.
Lucki NC, Sewer MB. The interplay between bioactive sphingolipids and steroid hormones. Steroids. 2010;75:390–9.
Santana P, Llanes L, Hernandez I, Gonzalez-Robayna I, Tabraue C, Gonzalez-Reyes J, et al. Interleukin-1 beta stimulates sphingomyelin hydrolysis in cultured granulose cells: evidence for a regulatory role of ceramide on progesterone and prostaglandin biosynthesis. Endocrinol. 1996;137:2480–9.
Lucki NC, Li D, Bandyopadhyay S, Wang E, Merrill AH, Sewer MB. Acid ceramidase (ASAH1) represses steroidogenic factor 1-dependent gene transcription in H295R human adrenocortical cells by binding to the receptor. Mol Cell Biol. 2012;32:4419–31.
Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–56.
Emoto K, Umeda M. An essential role for a membrane lipid in cytokinesis. Regulation of contractile ring disassembly by redistribution of phosphatidylethanolamine. T J Cell Biol. 2000;12:1215–24.
Steenbergen R, Nanowski TS, Beigneux A, Kulinski A, Young SG, Vance JE. Disruption of the phosphatidylserine decarboxylase gene in mice causes embryonic lethality and mitochondrial defects. J Biol Chem. 2005;2:40032–40.
Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, et al. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;14:656–67.
Acknowledgments
This work was supported by CNPq (National Council for Scientific and Technological Development), Brazil and by grant 2012/06389-4, São Paulo Research Foundation (FAPESP).
Conflicts of interest
All authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule Follicular fluid lipid profile by ESI/MS.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 264 kb)
Rights and permissions
About this article
Cite this article
Cordeiro, F.B., Cataldi, T.R., do Vale Teixeira da Costa, L. et al. Follicular fluid lipid fingerprinting from women with PCOS and hyper response during IVF treatment. J Assist Reprod Genet 32, 45–54 (2015). https://doi.org/10.1007/s10815-014-0375-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0375-0