Abstract
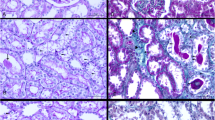
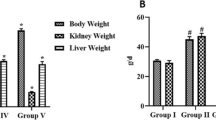
In the present study, it was aimed to investigate the effects of safranal, one of the components of saffron plant, on the inflammation in the rats in which experimental type 2 diabetes and obesity were formed. Type 2 diabetes is a disease characterized by insulin resistance and β-cell dysfunction. Therefore, in the present study, high-fat diet (HFD) and streptozotocin were used for being able to create experimental type 2 diabetes. In the first 6 weeks of the study, experimental groups were formed in five groups, after the stage of creating insulin resistance. The study groups were designed as control, HFD, HFD-Saf, DYB, and DYB-Saf groups. Safranal treatment was applied to the treatment groups for a period of 4 weeks. Throughout the study period (10 weeks), the weight gains and plasma glucose levels of the rats were determined each week and bi-weekly, respectively. At the end of the study, interferon gamma (IFN-γ), interleukin (IL)-1β, IL-6, tumor necrosis factor alpha (TNF-α), TAS and TOS levels in the pancreas and plasma were measured. In addition, the insulin and leptin levels in the plasma were determined. It was ascertained that, compared to the diabetic group, safranal decreased the inflammation both in the plasma and pancreas tissue, by reducing the TNF-α and IL-1β levels in particular. In addition, safranal was also found to decrease the oxidative stress increased due to type 2 diabetes in the plasma and pancreas tissue. It was concluded that safranal might be helpful in terms of reduction of diabetic complications, by means of its effects on both oxidative stress and inflammation, and that further studies should be carried out for this purpose.


Similar content being viewed by others
REFERENCES
IDF (International Diabetes Fedaration). 2014. IDF Diabetes atlas, Sixth edition, 12.
Ulukaya, E. 2007. (Translation editor) Lippincott’s illustrated reviews: biochemistry, 3rd ed. Turkey: Nobel Tıp Kitabevleri.
Baxter, M. 2008. Treatmant of type 2 diabetes: a structured management plan. Advances in Therapy 25(2): 106–114.
Negbi, M., B. Dagan, A. Dror, and D. Basker. 1989. Growth, flowering, vegetative reproduction and dormancy in the saffron crocus (Crocus sativus L.). Israel Journal of Botany 38: 95–113.
Lozano, P., D. Delgado, D. Gómez, M. Rubio, and J.L. Iborra. 2000. A non-destructive method to determine the safranal content of saffron (Crocus sativus L.) by supercritical carbon dioxide extraction combined with high-performance liquid chromatography and gas chromatography. Journal of Biochemical and Biophysical Methods 43(1–3): 367–378.
Cossignani, L., E. Urbani, M.S. Simonetti, A. Maurizi, C. Chiesi, and F. Blasi. 2014. Characterisation of secondary metabolites in saffron from central Italy (Cascia, Umbria). Food Chemistry 15(143): 446–451.
Kainbakht, S., and R. Hajiaghaee. 2011. Anti-hyperglycemic effects of saffron and its active constituents, crosin and safranal, in alloxan-induced diabetic rats. Journal of Medicinal Plant 10(39): 82–89.
Assimopoulou, A.N., Z. Sinakos, and V.P. Papageorgiou. 2005. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytotherapy Research 19: 997–1000.
Samarghandian, S., R. Afshari, A. Sadati. 2014. Evaluation of lung and bronchoalveolar lavage fluid oxidative stress indices for assessing the preventing effects of safranal on respiratory distress in diabetic rats. The Scientific World Journal, Article ID 251378, 6 pages http://dx.doi.org/10.1155/251378.
Mehdizadeh, R., M.R. Parizadeh, A.R. Khooei, S. Mehri, and H. Hosseinzadeh. 2013. Cardioprotective effect of saffron extract and safranal in isoproterenol-induced myocardial infarction in Wistar rats. Iranıan Journal of Basıc Medical Sciences 16(1): 56–63.
Escribano, J., G.L. Alonso, M. Coca-Prados, and J.A. Fernandez. 1996. Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Letters 100: 23–30.
Imenshahidi, M., H. Hosseinzadeh, and Y. Javadpour. 2010. Hypotensive effect of aqueous saffron extract (Crocus sativus L.) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytotherapy Research 24(7): 990–994.
Hazman, O. 2011. Investigation of the effect of oral antidiabetic agent sitagliptin on oxidant-antioxidant balance in rats with type 2 diabetes mellitus. Turkey: Depertmant of Biochemistry, PhD Thesis, Institue of Healt Sciences, Afyon Kocatepe Universty.
Zhang, M., M.Y. Lv, J. Lı, Z.G. Xu, L. Chen. 2008. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Experimental Diabetes Research, Article ID 704045, 1–9.
Erel, Ö. 2004. A novel automated method to measure total antioxidant response against potent free radical reactions. Clinical Biochemistry 37(2): 112–119.
Erel, Ö. 2005. A new automated colorimetric method for measuring total oxidant status. Clinical Biochemistry 38(12): 1103–1111.
Gutierrez, D.A., M.J. Puglisi, and A.H. Hasty. 2009. Impact of increased adipose tissue mass on inflammation, insulin resistance, and dyslipidemia. Current Diabetes Reports 9(1): 26–32.
Garcia, C., B. Feve, P. Ferré, S. Halimi, H. Baizri, L. Bordier, G. Guiu, O. Dupuy, B. Bauduceau, and H. Mayaudon. 2010. Diabetes and inflammation: fundamental aspects and clinical implications. Diabetes & Metabolism 36(5): 327–338.
Fronczyk, A., P. Molęda, K. Safranow, W. Piechota, and L. Majkowska. 2014. Increased concentration of C-reactive protein in obese patients with type 2 diabetes is associated with obesity and presence of diabetes but not with macrovascular and microvascular complications or glycemic control. Inflammation 37(2): 349–357.
Kim, Y.J., and T. Park. 2008. Genes are differentially expressed in the epididymal fat of rats rendered obese by a high-fat diet. Nutrition Research 28: 414–422.
Woods, S.C., R.J. Seeley, P.A. Rushing, D. D’Alessio, and P. Tso. 2003. A controlled high-Fat diet induces an obese syndrome in rats. The Journal of Nutrition 133: 1081–1087.
Lauterio, T.J., J.P. Bond, and E.A. Ulman. 1994. Development and characterization of a purified diet to identify obesity-susceptible and resistant rat populations. The Journal of Nutrition 124: 2172–2178.
Reed, M.J., K. Meszaros, L.J. Entes, M.D. Claypool, J.G. Pinkett, T.M. Gadbois, and G.M. Reaven. 2000. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism 49(11): 1390–1394.
Hazman, Ö., and S. Çelik. 2014. Effects of oral anti-diabetic agent sitagliptin on total antioxidant and oxidant status in rats with type 2 diabetes mellitus. Journal of Applied Biological Sciences 8(1): 31–37.
Melloul, D. 2008. Role of NFkB in β-cell death. Biochemical Society Transactions 36: 334–339.
Napetschnig, J., and H. Wu. 2013. Molecular basis of NF-κB signaling. Annual Review of Biophysics 42: 443–468.
Schoonbroodt, S., and J. Piette. 2000. Oxidative stress interference with the nuclear factor-kB activation pathways. Biochemical Pharmacology 60: 1075–1083.
Bowie, A., and L.A. O’Neill. 2000. Oxidative stress and nuclear factor-kappa B activation: a reassessment of the evidence in the light of recent discoveries. Biochemical Pharmacology 59(1): 13–23.
Maeda, A., K. Kai, M. Ishii, T. Ishii, and M. Akagawa. 2014. Safranal, a novel protein tyrosine phosphatase 1B inhibitor, activates insulin signaling in C2C12 myotubes and improves glucose tolerance in diabetic KK-Ay mice. Molecular Nutrition Food Research 58: 1177–1189.
Conflict of Interest
We declare that this study was financially supported by Scientific Research Projects Committee (project number 12.FENBIL.07), Rectorate of Afyon Kocatepe University, Afyonkarahisar, Turkey.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hazman, Ö., Ovalı, S. Investigation of the Anti-Inflammatory Effects of Safranal on High-Fat Diet and Multiple Low-Dose Streptozotocin Induced Type 2 Diabetes Rat Model. Inflammation 38, 1012–1019 (2015). https://doi.org/10.1007/s10753-014-0065-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-0065-1