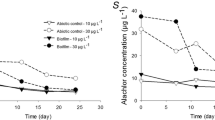
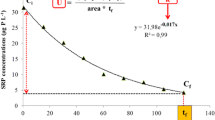
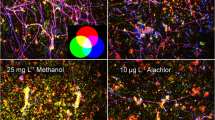
Abstract
Developing new biological indicators for monitoring toxic substances is a major environmental challenge. Intensive agricultural areas are generally pesticide-dependent and generate water pollution due to transfer of pesticide residues through spray-drift, run-off and leaching. The ecological effects of these pollutants in aquatic ecosystems are broad-ranging owing to the variety of substances present (herbicides, fungicides, insecticides, etc.). Biofilms (or periphyton) are considered to be early warning systems for contamination detection and their ability to reveal effects of pollutants led researchers to propose a variety of methods to detect and assess the impact of pesticides. The present article sought to provide new insights into the ecological significance of biofilm microbial communities and to discuss their bioindication potential for water quality and land use by reporting on 4 years of research performed on the French Ardières-Morcille experimental watershed (AMEW). Various biological indicators have been applied during several surveys on AMEW, allowing the characterisation of (i) the structure and diversity of biofilm communities [community level finger printing (CLFP) such as PCR–DGGE and pigment classes], (ii) functions associated with biofilm [community level physiological profiles (CLPP) such as extracellular enzymes, pesticides biodegradation or carbon sources biodegradation] and (iii) biofilm tolerance assessment (pollution-induced community tolerance, PICT) of the main contaminant in the AMEW (copper and diuron). Approaches based on CLFPs and PICT were consistent with each other and indicated the upstream–downstream impact due to the increasing land use by vineyards and the adaptation of algal and bacterial communities to the pollution gradient. CLPPs gave a contrasted bioindication because some parameters (most of the tested extracellular enzymes activities) did not detect a pollution gradient. Such CLPPs, CLFPs and PICT methods applied to biofilm could constitute the basis for a relevant in situ assessment both for chemical effects and aquatic ecosystem resilience.









Similar content being viewed by others
References
Admiraal, W., H. Blanck, M. Buckert De Jong, H. Guasch, N. Ivorra, V. Lehman, B. A. H. Nyström, M. Paulsson & S. Sabater, 1999. Short term toxicity of zinc to microbenthic algae and bacteria in a metal polluted stream. Water Research 33: 1989–1996.
AFNOR, 2004. Détermination de l’Indice Biotique Global Normalisé (IBGN). NF T 90-350, Association française de normalisation.
Barranguet, C., F. P. van den Ende, M. Rutgers, A. M. Breure, M. Greijdanus, J. J. Sinke & W. Admiraal, 2003. Copper-induced modifications of the trophic relations in riverine algalbacterial biofilms. Environmental Toxicology and Chemistry 22: 1340–1349.
Battin, T. J., L. A. Kaplan, D. Newbold & C. M. E. Hansen, 2003a. Contributions of microbial biofilms to ecosystem processes in stream mesocosms. Nature 426: 439–442.
Battin, T. J., L. A. Kaplan, D. Newbold, J. D. Cheng & C. M. E. Hansen, 2003b. Effects of current velocity on the nascent architecture of stream microbial biofilms. Applied and Environmental Microbiology 69: 5443–5452.
Bérard, A., U. Dorigo, J. F. Humbert, C. Leboulanger & F. Seguin, 2002. La méthode PICT (Pollution-Induced Community Tolerance) appliquée aux communautés algales. Intérêt comme outil de diagnose et d’évaluation du risque écotoxicologique en milieu aquatique. Annales de Limnologie 38: 247–261.
Bérard, A., U. Dorigo, I. Mercier, K. Becker van-Slooten & C. Leboulanger, 2003. Comparison of the ecotoxicological impact of triazines Irgarol 1051 and atrazine on microalgal cultures and natural microalgal communities in Lake Geneva. Chemosphere 53: 935–944.
Bernhard, E. & G. E. Likens, 2004. Controls on periphyton biomass in heterotrophic streams. Freshwater Biology 49: 14–27.
Blanck, H., S. A. Wängberg & S. Molander, 1988. Pollution-induced community tolerance—a new ecotoxicological tool. In Cairns, J. & J. R. Pratt (eds), Functional Testing of Aquatic Biota for Estimating Hazards of Chemicals. ASTM STP, Philadelphia: 219–230.
Boivin, M. E. Y., B. Massieux, A. M. Breure, F. P. van den Ende, G. D. Greve, M. Rutgers & W. Admiraal, 2005. Effects of copper and temperature on aquatic bacterial communities. Aquatic Toxicology 71: 345–356.
Boivin, M. E. Y., G. D. Greve, J. V. Garcia-Meza, B. Massieux, W. Sprenger, M. H. S. Kraak, A. M. Breure, M. Rutgers & W. Admiraal, 2007. Algal-bacterial interactions in metal contaminated floodplain sediments. Environmental Pollution 145(3): 884–894.
Boshker, H. T. S. & T. E. Cappenberg, 1998. Patterns of extracellular enzyme activities in littoral sediments of Lake Gooimeer, The Netherlands. FEMS Microbial Ecology 25(1): 79–86.
Campbell, C. D., S. J. Chapman, C. M. Cameron, M. S. Davidson & J. M. Potts, 2003. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Applied and Environmental Microbiology 69(6): 3593–3599.
Chappel, K. R. & R. Goulder, 1994. Enzymes as river pollutants and the response of native epilithic extracellular-enzyme activity. Environmental Pollution 86: 161–169.
Chrost, R. J., 1990. Microbial ectoenzymes in aquatic environments. In Overbeck, J. (ed.), Aquatic Microbial Ecology, Biochemical and Molecular Approach. Brock/Springer Series in Contemporary BioScience.
Collectif, 2008. Relations entre structures paysagères, transferts hydriques et flux géochimiques, état écologique des milieux aquatiques, Rapport final du programme ECOGER-Papier, INRA-Cemagref, Coord: Grimaldi, C. & B. Montuelle: 33 pp.
Dale, V. H. & H. C. Beyeler, 2001. Challenges in the development and use of biological indicators. Ecological Indicators 1: 3–10.
DCE, 2005. Définition du bon état des eaux, constitution des nouveaux référentiels et des modalités d’évaluation de l’état des eaux douces de surface. Ministère de l’Ecologie et du Développement Durable (ed.): 17 pp.
DeLorenzo, M. E., P. Scott & P. E. Ross, 2001. Toxicity of pesticides to aquatic microorganisms: a review. Environmental Toxicology and Chemistry 20(1): 84–98.
deNicola, D. M., E. de Eyto, A. Wemaere & K. Irvine, 2006. Periphyton response to nutrient addition in 3 lakes of different benthic productivity. Journal of North American Benthological Society 25: 616–631.
Dorigo, U., X. Bourrain, A. Berard & C. Leboulanger, 2004. Seasonal changes in the sensitivity of river microalgae to atrazine and isoproturon along a contamination gradient. Science of Total Environment 318: 101–104.
Dorigo, U., C. Leboulanger, A. Bérard, A. Bouchez, J. F. Humbert & B. Montuelle, 2007. Lotic biofilm community structure and pesticide tolerance along a contamination gradient in a vineyard area. Aquatic Microbial Ecology 50: 91–102.
Dorigo, U., M. Lefranc, C. Leboulanger, B. Montuelle & J. F. Humbert, 2009. Influence of sampling strategy on the assessment of the impact of pesticides on periphytic microbial communities in a small river. FEMS Microbial Ecology 67: 491–501.
Eisman, F. & B. Montuelle, 1999. Microbial methods for contaminants effects assessment in sediment. Reviews of Environmental Contamination and Toxicology 159: 41–93.
Franz, S., R. Altenburger, H. Heilmeier & M. Schmitt-Janssen, 2008. What contributes to the sensitivity of microalgae to triclosan? Aquatic Toxicology 90: 102–108.
Fuerhacker, M., 2009. EU Water Framework Directive and Stockholm Convention: can we reach the targets for priority substances and persistent organic pollutants? Environmental Science and Pollution Research 16: 92–97.
Garland, J. L., 1996. Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization. Soil Biology and Biochemistry 28: 213–221.
Gold, C., A. Feurtet-Mazel, M. Coste & A. Boudou, 2003. Impacts of Cd and Zn on the development of periphytic diatom communities in artificial streams located along a river pollution gradient. Archives of Environmental Contamination and Toxicology 44: 189–197.
Guasch, H. & S. Sabater, 1998. Light history influences the sensitivity to atrazine in periphytic algae. Journal of Phycology 34: 233–241.
Guasch, H., N. Ivorra, V. Lehmann, M. Paulsson, M. Real & S. Sabater, 1998. Community composition and sensitivity of periphyton to atrazine in flowing waters: the role of environmental factors. Journal of Applied Phycology 10: 203–213.
Guasch, H., W. Admiraal & S. Sabater, 2003. Contrasting effects of organic and inorganic toxicants on freshwater periphyton. Aquatic Toxicology 64: 165–175.
Guasch, H., E. Navarro, A. Serra & S. Sabater, 2004. Phosphate limitation influences the sensitivity to copper in periphytic algae. Freshwater Biology 49: 463–473.
Guasch, H., V. Lehmann, B. van Beusekom, S. Sabater & W. Admiraal, 2007. Influence of phosphate on the response of periphyton to atrazine exposure. Archives of Environmental Contamination and Toxicity 52: 32–37.
Harbott, E. L. & M. R. Grace, 2005. Extracellular enzyme response to bioavailability of dissolved organic C in streams of varying catchment urbanization. Journal of the North American Benthological Society 24: 588–601.
Haury, J., M. C. Peltre, M. Trémolières, J. Barbe, G. Thiébaut, I. Bernez, H. Daniel, P. Chatenet, G. Haan-Archipof, S. Muller, A. Dutartre, C. Laplace-Treyture, A. Cazaubon & E. Lambert-Servien, 2006. A new method to assess water trophy and organic pollution—the Macrophytes Biological Index for Rivers (IBMR): its application to different types of river and pollution. Hydrobiologia 570: 153–158.
Hill, B. H., A. T. Herlihy, P. R. Kaufmann, R. J. Stevenson, F. H. Mc Cormick & C. Burch Johnson, 2000. Use of periphyton assemblage data as an index of biotic integrity. Journal of North American Benthological Society 19: 50–67.
Hussain, S., T. Siddique, M. Saalem, M. Arshad & A. Khalid, 2009. Impact of pesticides on soil microbial diversity, enzymes and biochemical reactions. Advances in Agronomy 102: 159–200.
Lagacherie, P., O. Diot, N. Domange, V. Gouy, C. Floure, C. Kao, R. Moussa, J. M. Robbez-Masson & V. Szleper, 2006. An indicator approach for describing the spatial variability of artificial stream networks in regard with herbicide pollution in cultivated watersheds. Ecological Indicators 6: 265–279.
Landry, D., S. Dousset & F. Andreux, 2004. Laboratory leaching studies of oryzalin and diuron through three undisturbed vineyard soil columns. Chemosphere 54: 734–742.
Lenoir, A. & M. Coste, 1996. Development of a practical diatom index of overall water quality applicable to the French national water Board network. In Whitton, B. A. & E. Rott (eds), Use of Algae for Monitoring Rivers II. Studia Student. G.m.b.H, Innsbruck, Austria: 29–43.
Lopez, L., C. Pozo, B. Rodelas, C. Calvo & J. Gonzalez-Lopez, 2009. Influence of pesticides and herbicides presence on phosphatase activity and selected bacterial microbiota of a natural lake system. Ecotoxicology 15: 487–493.
Lyautey, E., S. Tessier, J. Y. Charcosset, J. L. Rols & F. Garabetian, 2003. Bacterial diversity of epilithic biofilm assemblages of an anthropised river section, assesed by DGGE analysis of a 16S rDNA fragment. Aquatic Microbial Ecology 33: 217–224.
Lyautey, E., C. R. Jackson, J. Cayrou, J. L. J. Rols & F. Garabetian, 2005. Bacterial community succession in natural river biofilm assemblages. Microbial Ecology 50: 589–601.
Marzorati, M., L. Wittebolle, N. Boon, D. Dofonchio & W. Verstraete, 2008. How to get more out of molecular fingerprints: practical tools for microbial ecology. Environmental Microbiology 10: 1571–1581.
McClellan, K., R. Altenburger & M. Schmitt-Janssen, 2008. Pollution-induced community tolerance as a measure of species interaction in toxicity assessment. Journal of Applied Ecology 45: 1514–1522.
Montuelle, B. & B. Volat, 1998. Impact of wastewater treatment plant discharge on enzyme activity in sediments. Ecotoxicology and Environmental Safety 40: 154–159.
Morin, S., M. Vivas-Nogues, T. T. Duong, A. Boudou, M. Coste & F. Delmas, 2007. Dynamics of benthic diatom colonization in a cadmium/zinc-polluted river (Riou-Mort, France). Fundamental and Applied Limnology 168: 179–187.
Morin, S., S. Pesce, A. Tlili, M. Coste & B. Montuelle, 2010. Recovery potential of periphytic communities in a river impacted by a vineyard watershed. Ecological Indicators 10: 419–426.
Othoniel, C., 2007. La croissance du biofilm photosynthétique: un indicateur du statut trophique des rivières? PhD Thesis, University of Bordeaux I: 245 pp.
Pérès, F., D. Florin, T. Grollier, A. Feurtet-Mazel, M. Coste, F. Ribeyre, M. Ricard & A. Boudou, 1996. Effects of the phenylurea herbicide isoproturon on periphytic diatom communities in freshwater indoor microcosm. Environmental Pollution 94: 141–152.
Pesce, S., C. Fajon, C. Bardot, F. Bonnemoy, C. Portelli & J. Bohatier, 2006. Effects of the phenylurea herbicide diuron on natural riverine microbial communities in an experimental study. Aquatic Toxicology 78: 303–314.
Pesce, S., C. Bardot, A. C. Lehours, I. Batisson, J. Bohatier & C. Fajon, 2008. Effects of diuron in microcosms on natural riverine bacterial community composition: new insight into phylogenetic approaches using PCR-TTGE analysis. Aquatic Sciences 70: 410–418.
Pesce, S., F. Martin-Laurent, N. Rouard & B. Montuelle, 2009a. Potential for microbial diuron mineralisation in a small wine-growing watershed: from treated plots to lotic receiver hydrosystem. Pest Management Science 65: 651–657.
Pesce, S., I. Batisson, C. Bardot, C. Fajon, C. Portelli, B. Montuelle & J. Bohatier, 2009b. Response of spring and summer riverine microbial communities following glyphosate exposure. Ecotoxicology and Environmental Safety 72: 1905–1912.
Pesce, S., C. Margoum & B. Montuelle, 2010. In situ relationships between spatio-temporal variations in diuron concentrations and phototrophic biofilm tolerance in a contaminated river. Water Research. doi:10.1016/j.watres.2009.11.053.
Rabiet, M., C. Margoum, V. Gouy, N. Carluer & M. Coquery, 2008. Transfert des pesticides et métaux dans un petit bassin versant viticole. Etude préliminaire de l’influence des conditions hydrologiques sur le transport de ces contaminants. Ingénieries EAT«Azote, phosphore et pesticides: stratégies et perspectives de réduction des flux»: 65–76.
Rabiet, M., C. Margoum, V. Gouy, N. Carluer & M. Coquery, 2010. Assessing pesticide concentrations and fluxes in the stream of a small vineyard catchment—effect of sampling frequency. Environmental Pollution 158: 737–748.
Ricart, M., H. Guasch, D. Barcelo, A. Geiszinger, M. Lopez de Alda, A. M. Romani, G. Vidal, M. Villagras & S. Sabater, 2009. Effects of low concentrations of the phenylurea herbicide diuron on biofilm algae and bacteria. Chemosphere 76: 1392–1401.
Rier, S. T. & R. J. Stevenson, 2002. Effects of light, dissolved organic carbon, and inorganic nutrients on the relationship between algae and heterotrophic bacteria in stream periphyton. Hydrobiologia 489: 179–194.
Romani, A. M. & S. Sabater, 2000. Variability of heterotrophic activity in Mediterranean stream biofilms: a multivariate analysis of physical–chemical and biological factors. Aquatic Sciences 6: 205–215.
Romani, A. M., H. Guasch, I. Munoz, J. Ruana, E. Vilalta, T. Schwartz, F. Emtlazi & S. Sabater, 2004. Biofilm structure and function and possible implications for riverine DOC dynamics. Microbial Ecology 47: 316–328.
Rosemond, A. D., P. Mulholland & S. Brawley, 2000. Seasonally shifting limitation of stream periphyton: response of algal populations and assemblage biomass and productivity to variation in light, nutrients and herbivores. Canadian Journal of Fisheries and Aquatic Science 57: 66–75.
Rutgers, M., I. M. Van’t Verlaat, B. Wind, L. Posthuma & A. M. Breure, 1998. Rapid method for assessing pollution-induced community tolerance in contaminated soil. Environmental Toxicology and Chemistry 17: 2210–2213.
Sabater, S., 2000. Diatom communities are indicators of environmental stress in the Guadiamar river, S-W Spain, following a major mine tailings spill. Journal of Applied Phycology 12: 113–124.
Sabater, S., H. Guasch, M. Ricart, A. Romani, G. Vidal, C. Klünder & M. Schmitt-Jansen, 2007. Monitoring the effect of chemical on biological communities: the biofilm as an interface. Analytical and Bioanalytical Chemistry 387: 1425–1434.
Schmitt, H., B. Martinali, P. Van Beelen & W. Seinen, 2006. On the limits of toxicant-induced tolerance testing: co tolerance and response variation of antibiotic effects. Environmental Toxicology and Chemistry 25: 1961–1968.
Schmitt-Jansen, M. & R. Altenburger, 2005. Community-level microalgal toxicity assessment by multiwavelength-excitation PAM fluorometry. Aquatic Toxicology 86: 49–58.
Schmitt-Jansen, M., U. Veit, G. Dudel & R. Altenburger, 2008. An ecological perspective in aquatic ecotoxicology: approaches and challenges. Basic and Applied Ecology 9: 337–345.
Sinsabaugh, R. L., M. M. Carreiro & D. A. Repert, 2002. Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 60: 1–24.
Stevenson, R. J. & Y. P. Pan, 1999. Assessing environmental conditions in rivers and streams with diatoms. In Stoermer, E. F. & J. P. Smol (eds), The Diatoms: Applications for the Environmental and Earth Sciences. Cambridge University Press, UK: 11–40.
Teissier, S. & M. Torre, 2002. Simultaneous assessment of nitrification and denitrification on freshwater epilithic biofilms by acetylene block method. Water Research 36: 3803–3811.
Tlili, A., U. Dorigo, B. Montuelle, C. Margoum, N. Carluer, V. Gouy, A. Bouchez & A. Bérard, 2008. Responses of chronically contaminated biofilms to short pulses of diuron. An experimental study simulating flooding events in a small river. Aquatic Toxicology 87: 252–263.
Toräng, L., N. Nyholm & H. J. Albrechtsen, 2003. Shifts in biodegradation kinetics of the herbicide MCPP and 2, 4-D at low concentrations in aerobic aquifer materials. Environmental Science and Technology 37: 3095–3103.
Villeneuve, A., 2008. Effets conjoints de facteurs physiques et chimiques sur la structure et la composition du périphyton: une approche multi échelle, PhD Thesis, U. de Savoie: 223 pp.
Villeneuve, A., B. Montuelle & A. Bouchez, 2010. Effect of minor changes in light intensity, current velocity and turbulence on the structure and function of the periphyton. Aquatic Sciences 72: 33–44.
Vu, S. H., S. Ishihara & K. Watanabe, 2006. Exposure risk assessment and evaluation of the best management practice for controlling pesticide runoff from paddy fields. Part 1: paddy watershed monitoring. Pesticides Management Science 62: 1193–1206.
Acknowledgements
The authors thank the two anonymous reviewers for improving the manuscript. They also thank Marjorie Maréchal for Microresp® assays; Laurence Blanc for extracellular enzymes measurements; Bernard Motte for field surveys; Christelle Margoum, Josiane Gahou, Céline Guillemain and Marina Coquery for chemical analysis and for the Morcille river database. The Ardières-Morcille experimental watershed is supported by the LTER Rhône Basin (ZABR). English was checked by ATT Scientific and Technical Translation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: R. J. Stevenson, S. Sabater / Global Change and River Ecosystems – Implications for Structure, Function and Ecosystem Services
Rights and permissions
About this article
Cite this article
Montuelle, B., Dorigo, U., Bérard, A. et al. The periphyton as a multimetric bioindicator for assessing the impact of land use on rivers: an overview of the Ardières-Morcille experimental watershed (France). Hydrobiologia 657, 123–141 (2010). https://doi.org/10.1007/s10750-010-0105-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0105-2