Abstract
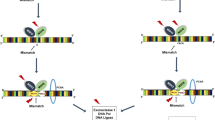
Germline mutations of DNA mismatch repair (MMR) genes predispose Lynch syndrome mutation carriers to the development of MMR-deficient tumors. MMR-deficient tumors show high-level microsatellite instability (MSI-H) and are typically characterized by a comparatively favorable prognosis and the absence of distant organ metastasis. Lynch syndrome-associated cancers are characterized by a pronounced local anti-tumoral immune response and usually display dense lymphocyte infiltration. This finding strongly suggested that the immune system may play an active role in the surveillance and biology of these cancers. The progression of MMR deficient cancers seems to be triggered by mutations in microsatellite sequences within gene-encoding regions. These mutations may cause shifts of the translational reading frame and thus give rise to the generation of potentially immunogenic frameshift peptides (FSP) at the carboxy terminal end of the respective gene products. FSP-specific immune responses are thought to represent one major mechanism by which the host’s adaptive immune system can recognize and potentially control Lynch syndrome-associated MSI-H cancers. Consequently, vaccination with FSP antigens represent a promising approach for treatment of Lynch syndrome-associated cancers, potentially also suitable for tumor prevention in so far tumor-free Lynch syndrome germ line mutation carriers. This review will summarize the molecular mechanisms contributing to the immunological phenotype of MSI-H cancers. In addition, clinical perspectives will be discussed, focusing on MSI-H cancer-associated FSP antigens as potential targets for immune therapy approaches.

Similar content being viewed by others
References
Watson P, Lin KM, Rodriguez-Bigas MA et al (1998) Colorectal carcinoma survival among hereditary nonpolyposis colorectal carcinoma family members. Cancer 83:259–266
Popat S, Hubner R, Houlston RS (2005) Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 23:609–618
Kim H, Jen J, Vogelstein B, Hamilton SR (1994) Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol 145:148–156
Risio M, Reato G, di Celle PF et al (1996) Microsatellite instability is associated with the histological features of the tumor in nonfamilial colorectal cancer. Cancer Res 56:5470–5474
Dolcetti R, Viel A, Doglioni C et al (1999) High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol 154:1805–1813
Michael-Robinson JM, Biemer-Hüttmann A et al (2001) Tumour infiltrating lymphocytes and apoptosis are independent features in colorectal cancer stratified according to microsatellite instability status. Gut 48:360–366
Shia J, Ellis NA, Paty PB et al (2003) Value of histopathology in predicting microsatellite instability in hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer. Am J Surg Pathol 27:1407–1417
Smyrk TC, Watson P, Kaul K et al (2001) Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer 91:2417–2422
Galon J, Costes A, Sanchez-Cabo F et al (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960–1964
Phillips SM, Banerjea A, Feakins R et al (2004) Tumour-infiltrating lymphocytes in colorectal cancer with microsatellite instability are activated and cytotoxic. Br J Surg 91:469–475
Michel S, Benner A, Tariverdian M et al (2008) High density of FOXP3-positive T cells infiltrating colorectal cancers with microsatellite instability. Br J Cancer 99:1867–1873
Bauer K, Michel S, Reuschenbach M et al (2011) Dendritic cell and macrophage infiltration in microsatellite-unstable and microsatellite-stable colorectal cancer. Fam Cancer 10:557–565
Banerjea A, Ahmed S, Hands RE et al (2004) Colorectal cancers with microsatellite instability display mRNA expression signatures characteristic of increased immunogenicity. Mol Cancer 3:21
Bernal M, García-Alcalde F, Concha A et al (2012) Genome-wide differential genetic profiling characterizes colorectal cancers with genetic instability and specific routes to HLA class I loss and immune escape. Cancer Immunol Immunother 61:803–816
de Miranda NF, Goudkade D, Jordanova ES et al (2012) Infiltration of Lynch colorectal cancers by activated immune cells associates with early staging of the primary tumor and absence of lymph node metastases. Clin Cancer Res 18:1237–1245
Jiricny J (2006) The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol 7:335–346
Duval A, Hamelin R (2002) Genetic instability in human mismatch repair deficient cancers. Ann Genet 45:71–75
Markowitz S, Wang J, Myeroff L et al (1995) Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science 268:1336–1338
Ionov Y, Yamamoto H, Krajewski S et al (2000) Mutational inactivation of the proapoptotic gene BAX confers selective advantage during tumor clonal evolution. Proc Natl Acad Sci U S A 97:10872–10877
Markowitz SD, Roberts AB (1996) Tumor suppressor activity of the TGF-beta pathway in human cancers. Cytokine Growth Factor Rev 7:93–102
Woerner SM, Kloor M, von Knebel Doeberitz M et al (2006) Microsatellite instability in the development of DNA mismatch repair deficient tumors. Cancer Biomark 2:69–86
Townsend A, Ohlen C, Rogers M et al (1994) Source of unique tumour antigens. Nature 371:662
Saeterdal I, Bjørheim J, Lislerud K et al (2001) Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci U S A 98:13255–13260
Linnebacher M, Gebert J, Rudy W et al (2001) Frameshift peptide-derived T-cell epitopes: a source of novel tumor-specific antigens. Int J Cancer 93:6–11
Ripberger E, Linnebacher M, Schwitalle Y et al (2003) Identification of an HLA-A0201-restricted CTL epitope generated by a tumor-specific frameshift mutation in a coding microsatellite of the OGT gene. J Clin Immunol 23:415–423
Schwitalle Y, Linnebacher M, Ripberger E et al (2004) Immunogenic peptides generated by frameshift mutations in DNA mismatch repair-deficient cancer cells. Cancer Immun 4:14
Garbe Y, Maletzki C, Linnebacher M (2011) An MSI tumor specific frameshift mutation in a coding microsatellite of MSH3 encodes for HLA-A0201-restricted CD8+ cytotoxic T cell epitopes. PLoS ONE 6:e26517
Schwitalle Y, Kloor M, Eiermann S et al (2008) Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology 134:988–997
Bauer K, Nelius N, Reuschenbach M et al (2012) T cell responses against microsatellite instability-induced frameshift peptides and influence of regulatory T cells in colorectal cancer. Cancer Immunol Immunother 62:27–37
Ribic CM, Sargent DJ, Moore MJ et al (2003) Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 349:247–257
Sargent DJ, Marsoni S, Monges G et al (2010) Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 28:3219–3226
Sinicrope FA, Foster NR, Thibodeau SN et al (2011) DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst 103:863–875
Burn J, Gerdes AM, Macrae F et al (2011) Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 378:2081–2087
Czéh M, Loddenkemper C, Shalapour S et al (2010) The immune response to sporadic colorectal cancer in a novel mouse model. Oncogene 29:6591–6602
Reuschenbach M, Kloor M, Morak M et al (2010) Serum antibodies against frameshift peptides in microsatellite unstable colorectal cancer patients with Lynch syndrome. Fam Cancer 9:173–179
Marsh L, Coletta PL, Hull MA et al (2012) Altered intestinal epithelium-associated lymphocyte repertoires and function in ApcMin/+mice. Int J Oncol 40:243–250
Bicknell DC, Kaklamanis L, Hampson R et al (1996) Selection for beta 2-microglobulin mutation in mismatch repair-defective colorectal carcinomas. Curr Biol 6:1695–1697
Kloor M, Becker C, Benner A et al (2005) Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res 65:6418–6424
Kloor M, Michel S, Buckowitz B et al (2007) Beta2-microglobulin mutations in microsatellite unstable colorectal tumors. Int J Cancer 121:454–458
Dierssen JW, de Miranda NF, Ferrone S et al (2007) HNPCC versus sporadic microsatellite-unstable colon cancers follow different routes toward loss of HLA class I expression. BMC Cancer 7:33
Chang CC, Ferrone S (2007) Immune selective pressure and HLA class I antigen defects in malignant lesions. Cancer Immunol Immunother 56:227–236
Koelzer VH, Baker K, Kassahn D et al (2012) Prognostic impact of β-2-microglobulin expression in colorectal cancers stratified by mismatch repair status. J Clin Pathol 65:996–1002
Tikidzhieva A, Benner A, Michel S et al (2012) Microsatellite instability and Beta2-Microglobulin mutations as prognostic markers in colon cancer: results of the FOGT-4 trial. Br J Cancer 106:1239–1245
Huang WC, Wu D, Xie Z et al (2006) Beta2-Microglobulin is a signaling and growth-promoting factor for human prostate cancer bone metastasis. Cancer Res 66:9108–9116
Josson S, Nomura T, Lin JT et al (2011) b2-Microglobulin induces epithelial to mesenchymal transition and confers cancer lethality and bone metastasis in human cancer cells. Cancer Res 71:2600–2610
Ma D, Luyten GP, Luyder TM et al (1995) Relationship between natural killer cell susceptibility and metastasis of human uveal melanoma cells in a murine model. Invest Ophthalmol Vis Sci 36:435–441
Jager MJ, Hurks HM, Levitskaya J et al (2002) HLA expression in uveal melanoma: there is no rule without some exception. Hum Immunol 63:444–451
Bernal M, Ruiz-Cabello F, Concha A et al (2012) Implication of the β2-microglobulin gene in the generation of tumor escape phenotypes. Cancer Immunol Immunother 61:1359–1371
Michel S, Linnebacher M, Alcaniz J et al (2010) Lack of HLA class II antigen expression in microsatellite unstable colorectal carcinomas is caused by mutations in HLA class II regulatory genes. Int J Cancer 127:889–898
Fearon ER (2011) Molecular genetics of colorectal cancer. Annu Rev Pathol 6:479–507
Kloor M, Huth C, Voigt AY et al (2012) Prevalence of mismatch repair-deficient crypt foci in Lynch syndrome: a pathological study. Lancet Oncol 13:598–606
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
von Knebel Doeberitz, M., Kloor, M. Towards a vaccine to prevent cancer in Lynch syndrome patients. Familial Cancer 12, 307–312 (2013). https://doi.org/10.1007/s10689-013-9662-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-013-9662-7