Summary
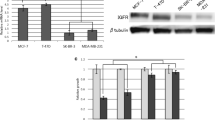
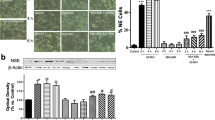
Growth hormone-releasing hormone (GHRH) and its receptors have been implicated in a variety of cellular phenotypes related with tumorigenesis process. Human epidermal growth factor receptor family members (HER) such as EGFR and HER2 are involved in mitogenic signaling pathways implicated in the progression of prostate cancer. We analyzed the cross-talk between GHRH and EGF receptors in prostate cancer. The effects of GHRH in HER signaling were evaluated on human androgen-independent PC3 prostate cancer cells in vitro and GHRH antagonist in vitro and in nude mice xenografts of PC3 prostate cancer. Time-course studies indicated that GHRH had a stimulatory activity on both the expression of EGFR and HER2. GHRH analogues, JMR-132 and JV-1–38, endowed with antagonistic activity for GHRH receptors, abrogated the response to GHRH in PC3 cells. GHRH stimulated a rapid ligand-independent activation of EGFR and HER2 involving at least cAMP/PKA and Src family signaling pathways. GHRH also stimulated a slow ligand-dependent activation of EGFR and HER2 involving an extracellular pathway with an important role for ADAM. Preliminary results also revealed an increase of mRNA for GHRH and GHRH receptor induced by EGF. The inhibition of tumor growth, in vivo, was associated with a substantial reduction in the expression of mRNA and protein levels of EGFR and HER2 in the tumors. GHRH antagonist JV-1–38, significantly decreased the phosphorylated Src levels. The cross-talk between HER and GHRH-R may be impeded by combining drugs acting upon GHRH receptors and HER family members in human advanced prostate cancer.







Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30
Baena E, Shao Z, Linn DE, Glass K, Hamblen MJ, Fujiwara Y et al (2013) ETV1 directs androgen metabolism and confers aggressive prostate cancer in targeted mice and patients. Genes Dev 27:683–698
Kuzumaki N, Suzuki A, Narita M, Hosoya T, Nagasawa A, Imai S et al (2012) Multiple analyses of G-protein coupled receptor (GPCR) expression in the development of gefitinib-resistance in transforming non-small-cell lung cancer. PLoS One 7:e44368
Schally AV, Varga JL, Engel JB (2008) Antagonists of growth-hormone-releasing hormone: an emerging new therapy for cancer. Nat Clin Pract Endocrinol Metab 4:33–43
Havt A, Schally AV, Halmos G, Varga JL, Toller GL, Horvath JE et al (2005) The expression of the pituitary growth hormone-releasing hormone receptor and its splice variants in normal and neoplastic human tissues. Proc Natl Acad Sci U S A 102:17424–17429
Rick FG, Schally AV, Szalontay L, Block NL, Szepeshazi K, Nadji M et al (2012) Antagonists of growth hormone-releasing hormone inhibit growth of androgen-independent prostate cancer through inactivation of ERK and Akt kinases. Proc Natl Acad Sci U S A 109:1655–1660
Stangelberger A, Schally AV, Djavan B (2008) New treatment approaches for prostate cancer based on peptide analogues. Eur Urol 53:890–900
Garcia-Fernandez MO, Schally AV, Varga JL, Groot K, Busto R (2003) The expression of growth hormone-releasing hormone (GHRH) and its receptor splice variants in human breast cancer lines; the evaluation of signaling mechanisms in the stimulation of cell proliferation. Breast Cancer Res Treat 77:15–26
Kanashiro CA, Schally AV, Zarandi M, Hammann BD, Varga JL (2004) Suppression of growth of H-69 small cell lung carcinoma by antagonists of growth hormone releasing hormone and bombesin is associated with an inhibition of protein kinase C signaling. Int J Cancer 112:570–576
Muñoz-Moreno L, Arenas MI, Schally AV, Fernández-Martínez AB, Zarka E, González-Santander M et al (2013) Inhibitory effects of antagonists of growth hormone-releasing hormone on growth and invasiveness of PC3 human prostate cancer. Int J Cancer 132:755–765
Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A (2001) The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr Relat Cancer 8:11–31
Sebastian S, Settleman J, Reshkin SJ, Azzariti A, Bellizzi A, Paradiso A (2006) The complexity of targeting EGFR signalling in cancer: from expression to turnover. Biochim Biophys Acta 1766:120–139
Di Lorenzo G, Tortora G, D’Armiento FP, De Rosa G, Staibano S, Autorino R et al (2002) Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin Cancer Res 8:3438–3444
Berger R, Lin DI, Nieto M, Sicinska E, Garraway LA, Adams H et al (2006) Androgen-dependent regulation of Her-2/neu in prostate cancer cells. Cancer Res 66:5723–5728
Delcourt N, Bockaert J, Marin P (2007) GPCR-jacking: from a new route in RTK signalling to a new concept in GPCR activation. Trends Pharmacol Sci 28:602–607
Sotomayor S, Carmena MJ, Schally AV, Sánchez-Chapado M, Prieto JC, Bajo AM (2007) Transactivation of HER2 by vasoactive intestinal peptide in experimental prostate cancer: antagonistic action of an analog of growth-hormone-releasing hormone. Int J Oncol 31:1223–1230
Sotomayor S, Muñoz-Moreno L, Carmena MJ, Schally AV, Sánchez-Chapado M, Prieto JC et al (2010) Regulation of her expression and transactivation in human prostate cancer cells by a targeted cytotoxic bombesin analog (AN-215) and a bombesin antagonist (RC-3095). Int J Cancer 127:1813–1822
Wheeler DL, Iida M, Dunn EF (2009) The role of Src in solid tumors. Oncologist 14:667–678
Araujo JC, Trudel GC, Paliwal P (2013) Long-term use of dasatinib in patients with metastatic castration-resistant prostate cancer after receiving the combination of dasatinib and docetaxel. Cancer Manag Res 6:25–30
Asim M, Siddiqui IA, Hafeez BB, Baniahmad A, Mukhtar H (2008) Src kinase potentiates androgen receptor transactivation function and invasion of androgen-independent prostate cancer C4-2 cells. Oncogene 27:3596–3604
Eguchi S, Numaguchi K, Iwasaki H, Matsumoto T, Yamakawa T, Utsunomiya H et al (1998) Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J Biol Chem 273:8890–8896
Liebmann C (2011) EGF receptor activation by GPCRs: an universal pathway reveals different versions. Mol Cell Endocrinol 331:222–231
Fernández-Martínez AB, Bajo AM, Isabel Arenas M, Sánchez-Chapado M, Prieto JC, Carmena MJ (2010) Vasoactive intestinal peptide (VIP) induces malignant transformation of the human prostate epithelial cell line RWPE-1. Cancer Lett 299:11–21
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S (eds) Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press, New Jersey, pp 365–386
Carlsson J, Shen L, Xiang J, Xu J, Wei Q (2013) Tendencies for higher co-expression of EGFR and HER2 and downregulation of HER3 in prostate cancer lymph node metastases compared with corresponding primary tumors. Oncol Lett 5:208–214
Rezaiemanesh A, Majidi J, Baradaran B, Movasaghpour A, Nakhlband A, Barar J et al (2010) Impacts of anti-EGFR monoclonal antibody in prostate cancer PC3 cells. Hum Antibodies 19:63–70
Bartlett JM, Brawley D, Grigor K, Munro AF, Dunne B, Edwards J (2005) Type I receptor tyrosine kinases are associated with hormone escape in prostate cancer. J Pathol 205:522–529
Valdehita A, Bajo AM, Schally AV, Varga JL, Carmena MJ, Prieto JC (2009) Vasoactive intestinal peptide (VIP) induces transactivation of EGFR and HER2 in human breast cancer cells. Mol Cell Endocrinol 302:41–48
De Heuvel E, Wallace L, Sharkey KA, Sigalet DL (2012) Glucagon-like peptide 2 induces vasoactive intestinal polypeptide expression in enteric neurons via phosphatidylinositol 3-kinase-γ signaling. Am J Physiol Endocrinol Metab 303:E994–E1005
Bertelsen LS, Barrett KE, Keely SJ (2004) Gs protein-coupled receptor agonists induce transactivation of the epidermal growth factor receptor in T84 cells: implications for epithelial secretory responses. J Biol Chem 279:6271–6279
Majeed N, Blouin MJ, Kaplan-Lefko PJ, Barry-Shaw J, Greenberg NM, Gaudreau P et al (2005) A germ line mutation that delays prostate cancer progression and prolongs survival in a murine prostate cancer model. Oncogene 24:4736–4740
Barabutis N, Schally AV (2008) Knocking down gene expression for growth hormone-releasing hormone inhibits proliferation of human cancer cell lines. Br J Cancer 98:1790–1796
Rekasi Z, Czompoly T, Schally AV, Boldizsar F, Varga JL, Zarandi M et al (2005) Antagonist of growth hormone-releasing hormone induces apoptosis in LNCaP human prostate cancer cells through a Ca2+-dependent pathway. Proc Natl Acad Sci U S A 102:3435–3440
Halmos G, Schally AV, Czompoly T, Krupa M, Varga JL, Rekasi Z (2002) Expression of growth hormone-releasing hormone and its receptor splice variants in human prostate cancer. J Clin Endocrinol Metab 87:707–4714
Csernus V, Schally AV, Groot K (1999) Antagonistic analogs of growth hormone releasing hormone (GHRH) inhibit cyclic AMP production of human cancer cell lines in vitro. Peptides 20:843–850
Delcourt N, Thouvenot E, Chanrion B, Galéotti N, Jouin P, Bockaert J, Marin P (2007) PACAP type I receptor transactivation is essential for IGF-1 receptor signalling and antiapoptotic activity in neurons. EMBO J 26:1542–1551
Fizazi K (2007) The role of Src in prostate cancer. Ann Oncol 18:1765–1773
Rozengurt E (2007) Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol 213:589–602
El Zein N, D’Hondt S, Sariban E (2010) Crosstalks between the receptors tyrosine kinase EGFR and TrkA and the GPCR, FPR, in human monocytes are essential for receptors-mediated cell activation. Cell Signal 22:1437–1447
Higashiyama S, Nanba D, Nakayama H, Inoue H, Fukuda S (2011) Ectodomain shedding and remnant peptide signalling of EGFRs and their ligands. J Biochem 150:15–22
Xiao LJ, Lin P, Lin F, Liu X, Qin W, Zou HF et al (2012) ADAM17 targets MMP-2 and MMP-9 via EGFR-MEK-ERK pathway activation to promote prostate cancer cell invasion. Int J Oncol 40:1714–1724
Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C et al (1999) EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402:884–888
Fischer OM, Hart S, Gschwind A, Ullrich A (2003) EGFR signal transactivation in cancer cells. Biochem Soc Trans 31:1203–1208
Edwards DR, Handsley MM, Pennington CJ (2008) The ADAM metalloproteinases. Mol Aspects Med 29:258–289
Maretzky T, Zhou W, Huang XY, Blobel CP (2011) A transforming Src mutant increases the bioavailability of EGFR ligands via stimulation of the cell-surface metalloproteinase ADAM17. Oncogene 30:611–618
Yen L, Benlimame N, Nie ZR, Xiao D, Wang T, Al Moustafa AE et al (2002) Differential regulation of tumor angiogenesis by distinct ErbB homo- and heterodimers. Mol Biol Cell 13:4029–4044
Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz G, Yan DH et al (2000) HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res 60:6841–6845
Mira E, Lacalle RA, González MA, Gómez-Moutón C, Abad JL, Bernad A et al (2001) A role for chemokine receptor transactivation in growth factor signaling. EMBO Rep 2:151–156
Buchholz S, Schally AV, Engel JB, Hohla F, Heinrich E, Koester F et al (2007) Potentiation of mammary cancer inhibition by combination of antagonists of growth hormone-releasing hormone with docetaxel. Proc Natl Acad Sci U S A 104:1943–1946
Xu W, Marcu M, Yuan X, Mimnaugh E, Patterson C, Neckers L (2002) Chaperone-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc Natl Acad Sci U S A 99:12847–12852
Citri A, Gan J, Mosesson Y, Vereb G, Szollosi J, Yarden Y (2004) Hsp90 restrains ErbB-2/HER2 signalling by limiting heterodimer formation. EMBO Rep 5:1165–1170
Hendriks BS, Opresko LK, Wiley HS, Lauffenburger D (2003) Quantitative analysis of HER2-mediated effects on HER2 and epidermal growth factor receptor endocytosis: distribution of homo- and heterodimers depends on relative HER2 levels. J Biol Chem 278:23343–23351
Acknowledgments
This work was supported by the Junta de Comunidades de Castilla-La Mancha [grant number PII10-0189-3222 to A.M.B.] and by the University of Alcalá [FPU/UAH grant to L.M.M.]. Synthesis of GHRH antagonists in the laboratory of AVS was supported by the Medical Research Service of the Veterans Affairs Department.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muñoz-Moreno, L., Arenas, M.I., Carmena, M.J. et al. Growth hormone-releasing hormone antagonists abolish the transactivation of human epidermal growth factor receptors in advanced prostate cancer models. Invest New Drugs 32, 871–882 (2014). https://doi.org/10.1007/s10637-014-0131-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0131-4