Abstract
Background
Marijuana may be used by some patients with gastroparesis (Gp) for its potential antiemetic, orexigenic, and pain-relieving effects.
Aims
The aim of this study was to describe the use of marijuana by patients for symptoms of Gp, assessing prevalence of use, patient characteristics, and patients’ perceived benefit on their symptoms of Gp.
Methods
Patients with symptoms of Gp underwent history and physical examination, gastric emptying scintigraphy, and questionnaires assessing symptoms. Patients were asked about the current use of medications and alternative medications including marijuana.
Results
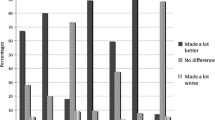
Fifty-nine of 506 (11.7%) patients with symptoms of Gp reported current marijuana use, being similar among patients with delayed and normal gastric emptying and similar in idiopathic and diabetic patients. Patients using marijuana were younger, more often current tobacco smokers, less likely to be a college graduate, married or have income > $50,000. Patients using marijuana had higher nausea/vomiting subscore (2.7 vs 2.1; p = 0.002), higher upper abdominal pain subscore (3.5 vs 2.9; p = 0.003), more likely to be using promethazine (37 vs 25%; p = 0.05) and dronabinol (17 vs 3%; p < 0.0001). Of patients using marijuana, 51% had been using it for more than 2 years, 47% were using this once or more per day, and 81% of marijuana users rated their benefit from marijuana as better or much better.
Conclusions
A subset of patients (12%) with symptoms of Gp use marijuana. Patients with severe nausea and abdominal pain were more likely to use marijuana and perceive it to be beneficial for their symptoms.
Trial Registration
ClinicalTrials.gov Identifier: NCT01696747.
Similar content being viewed by others
References
Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592–1622.
Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L, American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18–37.
Lee LA, Chen J, Yin J. Complementary and alternative medicine for gastroparesis. Gastroenterol Clin N Am. 2015;44:137–150.
Jehangir A, Parkman HP. Cannabinoid use in patients with gastroparesis and related disorders. Am J Gastroenterol.. 2019;114:945–953.
Sharkey KA, Wiley JW. The Role of the endocannabinoid system in the brain-gut axis. Gastroenterology. 2016;151:252–266.
Malik Z, Baik D, Schey R. The role of cannabinoids in the regulation of nausea and vomiting, and visceral pain. Curr Gastroenterol Rep. 2015;17:429–435.
Parkman HP, Hallinan EK, Hasler WL, Farrugia G, Koch KL, Nguyen L, Snape WJ, Abell TL, McCallum RW, Sarosiek I, Pasricha PJ, Clarke J, Miriel L, Tonascia J, Hamilton F; NIDDK Gastroparesis Clinical Research Consortium (GpCRC). Early satiety and postprandial fullness in gastroparesis correlate with gastroparesis severity, gastric emptying, and water load testing. Neurogastroenterol Motil 2017;29
Abell TL, Bernstein VK, Cutts T, et al. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil. 2006;18:263–283.
Rentz AM, Kahrilas P, Stanghellini V, et al. Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual Life Res. 2004;13:1737–1749.
Revicki DA, Rentz AM, Dubois D, et al. Development and validation of a patient-assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther. 2003;18:141–150.
De la Loge C, Trudeau E, Marquis P, et al. Cross-cultural development and validation of a patient self-administered questionnaire to assess quality of life in upper gastrointestinal disorders: the PAGI-QOL. Qual Life Res. 2004;13:1751–1762.
Ware JE, Kosinski M, Dewey JE. How to Score Version 2 of the SF-36®Health Survey. Lincoln, RI: QualityMetric Incorporated; 2000.
Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597.
Spielberger C, Gorsuch R, Lushene R. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1970.
Kocalevent RD, Hinz A, Brähler E. Standardization of a screening instrument (PHQ-15) for somatization syndromes in the general population. BMC Psychiatry. 2013;13:91.
Spiller RC, Humes DJ, Campbell E, et al. The Patient Health Questionnaire 12 Somatic Symptom scale as a predictor of symptom severity and consulting behaviour in patients with irritable bowel syndrome and symptomatic diverticular disease. Aliment Pharmacol Ther. 2010;32:811–820.
Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456–1462.
Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy. Am J Gastro. 2008;103:753–763.
Agresti A. Categorical data analysis. New York: John Wiley & Sons, Inc.; 1990.
Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19:716–723.
SAS Institute, Inc. SAS software, version 9.3 of the SAS system for Windows. Cary, NC, 2002-2010. StataCorp. 2011. Stata statistical software: release 12. College Station, TX: StataCorp LP.
National Survey on Drug Use and Health. https://nsduhweb.rti.org/respweb/homepage.cfm Visited site 3/2/2019
Bhandari A, Wagner T. Self-reported utilization of health care services: improving measurement and accuracy. Med Care Res Rev. 2006;63:217–235.
Choung RS, Locke GR 3rd, Lee RM, et al. Cyclic vomiting syndrome and functional vomiting in adults: association with cannabinoid use in males. Neurogastroenterol Motil. 2012;24:20-6, e1.
Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101–115.
Pasricha PJ, Colvin R, Yates K, et al. Characteristics of patients with chronic unexplained nausea and vomiting and normal gastric emptying. Clin Gastroenterol Hepatol. 2011;9:567–576.
Stanghellini V, Chan FK, Hasler WL, et al. Gastroduodenal Disorders. Gastroenterology. 2016;150:1380–1392.
Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut. 2004;53:1566–1570.
Pattathan MB, Hejazi RA, McCallum RW. Association of marijuana use and cyclic vomiting syndrome. Pharmaceuticals. 2012;29:719–726.
Hejazi RA, Lavenbarg TH, McCallum RW. Spectrum of gastric emptying patterns in adult patients with cyclic vomiting syndrome. Neurogastroenterol Motil. 2010;22:1298-302 e338.
Camilleri M. Cannabinoids and gastrointestinal motility: Pharmacology, clinical effects, and potential therapeutics in humans. Neurogastro Motility. 2018;30:e13370.
Bateman DN. Delta-9-tetrahydrocannabinol and gastric emptying. Br J Clin Pharmacol. 1983;15:749–751.
McCallum RW, Soykan I, Sridhar KR, Ricci DA, Lange RC, Plankey MW. Delta-9-tetrahydrocannabinol delays the gastric emptying of solid food in humans: a double-blind, randomized study. Aliment Pharmacol Ther. 1999;13:77–80.
Esfandyari T, Camilleri M, Ferber I, et al. Effect of a cannabinoid agonist of gastrointestinal transit and postprandial satiation in healthy human subjects: a randomized, placebo-controlled study. Neurogastroenterol Motil. 2006;18:831–838.
Meiri E, Jhangiani H, Vredenburgh JJ, et al. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr Med Res Opin. 2007;23:533–543.
Peters J, Chien J. Contemporary routes of cannabis consumption: a primer for clinicians. J Am Osteopath Assoc. 2018;118:67–70.
Cohen K, Weizman A, Weinstein A. Positive and negative effects of cannabis and cannabinoids on health. Clin Pharmacol Ther. 2019;105:1139–1147.
Turna J, Simpson W, Patterson B, Lucas P, van Ameringen M. Cannabis use behaviors and prevalence of anxiety and depressive symptoms in a cohort of Canadian medicinal cannabis users. J Psychiartr Res. 2019;111:134–139.
Di Forti M, Quattrone D, Freeman TP, et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. Lancet Psychiatry. 2019;6:427–436.
Funding
The NIH/NIDDK Gastroparesis Clinical Research Consortium (GpCRC) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK073975 [Parkman], U01DK073983 [Pasricha], U01DK074007 [Abell], U01DK073974 [Koch], U01DK074035 [McCallum], U01DK112193 [Kuo], and U01DK074008 [Tonascia]).
Author information
Authors and Affiliations
Consortia
Contributions
HPP was involved in the study conceptualization, patient recruitment, data interpretation, and writing of the manuscript; EPS contributed to the statistical analysis, data interpretation, and writing of the manuscript; LN was involved in the study conceptualization, patient recruitment, and revision of the manuscript; KY was involved in the statistical analysis, data interpretation, writing of the manuscript; TLA was involved in the study conceptualization, patient recruitment, and revision of the manuscript; WLH contributed to the study conceptualization, patient recruitment, and revision of the manuscript; WJS was involved in the study conceptualization, patient recruitment, revision of the manuscript; JC was involved in the patient recruitment and revision of the manuscript; RS contributed to the study conceptualization and revision of the manuscript; KLK was involved in the study conceptualization, patient recruitment, and revision of the manuscript; BK was involved in the patient recruitment and revision of the manuscript; RWM was involved in the study conceptualization, patient recruitment, and revision of the manuscript; IS was involved in the study conceptualization, patient recruitment, and revision of the manuscript; MG was involved in the study conceptualization and revision of the manuscript; GF was involved in the study conceptualization, revision of the manuscript; JT contributed to the study conceptualization, statistical analysis, data interpretation, and revision of the manuscript; PJP was involved in the study conceptualization, patient recruitment, and revision of the manuscript; FH contributed to the study conceptualization and revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Parkman, H.P., Sharkey, E.P., Nguyen, L.A. et al. Marijuana Use in Patients with Symptoms of Gastroparesis: Prevalence, Patient Characteristics, and Perceived Benefit. Dig Dis Sci 65, 2311–2320 (2020). https://doi.org/10.1007/s10620-019-05963-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05963-2