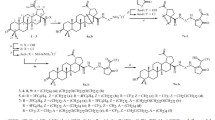
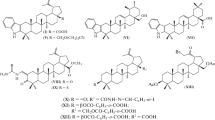
Conjugates of betulin and betulinic and betulonic acids with 2-aminoethane- and N-methyl-2-aminoethanesulfonic acids were synthesized for the first time and were interesting as potential biologically active compounds. Experiments in vitro in MDCK cell culture using the MTT assay found that betulin and betulinic-acid derivatives with aminoethanesulfonic acid bound to triterpene C-3 or C-28 through an ester linker were less toxic than the native compounds.
Similar content being viewed by others
References
P. A. Krasutsky, Nat. Prod. Rep., 23, 919 (2006).
R. Csuk, Expert Opin. Ther. Pat., 24 (8), 913 (2014).
P. Yogeeswari and D. Sriram, Curr. Med. Chem., 12 (6), 657 (2005).
H. Wang, R. Xu, Y. Shi, L. Si, P. Jiao, Z. Fan, X. Han, X. Wu, X. Zhou, F. Yu, Y. Zhang, D. Zhou, and S. Xiao, Eur. J. Med. Chem., 110, 376 (2016).
H. Ripps and W. Shen, Mol. Vision, 18, 2673 (2012).
N. Chen and J. Xu, Tetrahedron, 68 (11), 2513 (2012).
R. C. Gupta, T. Win, and S. Bittner, Curr. Med. Chem., 12 (17), 2021 (2005).
A. A. Shtro, A. V. Slita, L. A. Karpinskaya, A. V. Galochkina, and V. V. Zarubaev, Lechashchii Vrach, 10, 52 (2012).
S. Q. Yang, M. Froeyen, E. Lescrinier, P. Marliere, and P. Herdewijn, Org. Biomol. Chem., 9 (1), 111 (2011).
M. G. Mutchnick, M. N. Ehrinpreis, J. L. Kinzie, and R. R. Peleman, Antiviral Res., 24 (2–3), 245 (1994).
J. Yoon, A. Jekle, R. Najafi, F. Ruado, M. Zuck, B. Khosrovi, B. Memarzadeh, D. Debabov, L. Wang, and M. Anderson, Antiviral Res., 92 (3), 470 (2011).
T. P. Shiau, E. Low, B. Kim, E. D. Turtle, C. Francavilla, D. J. R. O’Mahony, L. Friedman, L. D’Lima, A. Jekle, D. Debabov, M. Zuck, N. J. Alvarez, M. Anderson, R. Najafi, and R. K. Jain, Bioorg. Med. Chem. Lett., 23 (20), 5650 (2013).
F. Kong, N. Zhao, M. M. Siegel, K. Janota, J. S. Ashcroft, F. E. Koehn, D. B. Borders, and G. T. Carter, J. Am. Chem. Soc., 120 (51), 13301 (1998).
Y. Takahashi, J.-R. Nakijiquinones, M. Ushio, T. Kubota, S. Yamamoto, J. Fromont, and J. Kobayashi, J. Nat. Prod., 73 (3), 467 (2010).
A. Aiello, E. Fattorusso, P. Luciano, A. Macho, M. Menna, and E. Munoz, J. Med. Chem., 48 (9), 3410 (2005).
A. Barthel, S. Stark, and R. Csuk, Tetrahedron, 64 (39), 9225 (2008).
D. S. H. L. Kim, Z. Chen, T. Nguyen, J. M. Pezzuto, S. Qiu, and Z.-Z. Lu, Synth. Commun., 27 (9), 1607 (1997).
N. V. Uzenkova, N. I. Petrenko, M. M. Shakirova, E. E. Shul’ts, and G. A. Tolstikov, Chem. Nat. Compd., 41, 692 (2005).
X. Y. Wang, S. Y. Zhang, J. Li, H. N. Liu, X. Xie, and F. J. Nan, Acta Pharm. Sin., 35, 1463 (2014).
J. W. Schick and E. F. Degering, Ind. Eng. Chem., 39 (7), 906 (1947).
D. G. I. Kingston and Z.-Y. Zhao, EP Pat. 0473326 A1, Mar. 4, 1992.
X. Fang, J. Ling, F. Liu, and Y. Chen, CN Pat. 102675160A, Sept. 19, 2012.
M. S. Yunusov, N. G. Komissarova, and N. G. Belenkova, RU Pat. No. 2,270,201, Feb. 20, 2006; Byull. Izobret., No. 5, 1 (2006).
H. Kommera, G. N. Kaluderovic, J. Kalbitz, and R. Paschke, Arch. Pharm. Chem. Life Sci., 343 (8), 449 (2010).
E. Borenfreund, H. Babich, and N. Martin-Alguacil, Toxicol. in Vitro, 2 (1), 1 (1988).
Acknowledgment
The work was supported financially by a grant from the Russian Science Foundation (Project No. 14-13-01307).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2017, pp. 772–778.
Rights and permissions
About this article
Cite this article
Komissarova, N.G., Dubovitskii, S.N., Shitikova, O.V. et al. Synthesis of Conjugates of Lupane-Type Pentacyclic Triterpenoids with 2-Aminoethane- and N-Methyl-2-Aminoethanesulfonic Acids. Assessment of in vitro Toxicity. Chem Nat Compd 53, 907–914 (2017). https://doi.org/10.1007/s10600-017-2153-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-2153-6