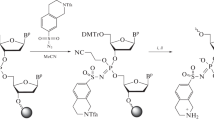
Abstract
Antisense oligonucleotides with iron binding hydroxamate linkages are designed to act as sequence-selective cleaving agents of complementary nucleic acids through Fenton chemistry. Oligothymidylate analogs with hydroxamate linkages were efficiently synthesized from coupling of nucleoside intermediates, activated as p-nitrophenyl carbonates, with hydroxylamine derivatized nucleosides. Iron binding studies showed that hydroxamate linked oligonucleotides are effective iron chelators when there are three nonadjacent internucleosidic hydroxamate linkages available in the same oligonucleotide molecule. However, analysis of the CD spectra of an oligothymidylate 16mer, which contained complete substitution of all phosphates with hydroxamates, indicated that the hydroxamate linkage was too rigid to allow the analog to base pair with the complementary DNA d(A16). Syntheses of mix-linked thymidine oligomers with up to three hydroxamate linkages incorporated in the center of the sequence are also reported. Iron binding of the thymidine oligomer with hydroxamate linkages was confirmed by matrix assisted laser desorption mass spectrometry analysis. Nuclease stability assays showed that the modified oligonucleotides have enhanced resistance toward nuclease S1 (endonuclease) compared to natural oligonucleotides. A thymidine 16mer with three hydroxamate linkages incorporated in the center of the sequence was shown to be able to bind with both iron and its complementary polyA strand. A small destablizing effect was observed when the phosphodiester linkage was changed to the hydroxamate linkage. Under Fenton chemistry conditions, this novel iron binding oligothymidylate analog cleaved the complementary DNA strand sequence-selectively.













Similar content being viewed by others
References
Aboul-Fadl T (2005) Antisense oligonucleotides: the state of the art. Curr Med Chem 12:2193–2214. doi:10.2174/0929867054864859
Agrawal S (ed) (1996) Antisense therapeutics. Humana Press, Totowa
Agrawal S (1999) Antisense oligonucleotides: a new therapeutic principle. Biochim Biophys Acta 1489:53–68
Arano Y, Uezono T, Akizawa H, Ono M, Wakisaka K, Nakayama M, Sakahara H, Konishi J, Yokoyama A (1996) Reassessment of diethylenetriaminepentaacetic acid (DTPA) as a chelating agent for Indium-111 labeling of polypeptides using a newly synthesized monoreactive DTPA derivative. J Med Chem 39:3451–3460. doi:10.1021/jm950949+
Chu CK, Baker DC (eds) (1993) Nucleosides and nucleotides as antitumor and antiviral agents. Plenum Press, New York
Crooke ST, Lebleu B (eds) (1993) Antisense research and applications. CRC Press, Boca Raton
Dionis JB, Jenny HB, Peter HH (1989) Synthesis and analytical characterization of a major desferrioxamine B metabolite. J Org Chem 54:5623–5627. doi:10.1021/jo00284a044
Dreyer GB, Dervan PB (1985) Sequence-specific cleavage of single-stranded DNA: oligodeoxynucleotide-EDTA × Fe(II). Proc Natl Acad Sci USA 82:968–972. doi:10.1073/pnas.82.4.968
Dubowchik GM, King HD, Pham-Kaplita K (1997) Efficient mitomycin C coupling with stable p-nitrophenyl-benzyl carbonates using n-hydroxybenzotriazole as a catalytic additive. Tetrahedron Lett 38:5261–5264. doi:10.1016/S0040-4039(97)01159-3
Eckstein F (ed) (1991) Oligonucleotides and analogues: a practical approach. IRL Press, Oxford
Fischman AJ, Babich JW, Strauss HW (1993) A ticket to ride: peptide radiopharmaceuticals. J Nucl Med 34:2253–2263
Floreancig PE, Swalley SE, Trauger JW, Dervan PB (1999) Recognition of the minor groove of DNA by hairpin polyamides containing α-substituted-β-amino acids. J Am Chem Soc 122:6342–6350. doi:10.1021/ja000509u
Ghosh M, Lambert LJ, Huber PW, Miller MJ (1995) Synthesis, Bioactivity, and DNA-Cleaving Ability of Desferrioxamine β-Nalidixic Acid and Anthraquinone Carboxylic Acid Conjugates. Bioorg Med Chem Lett 5:2337–2340. doi:10.1016/0960-894X(95)00412-M
Hashimoto S, Nakamur Y (1995) Nuclease activity of a hydroxamic acid derivative in the presence of various metal ions. J Chem Soc Chem Comm 1413–1414
Hayakawa Y, Hirose M, Noyori R (1993) O-Allyl protection of guanine and thymine residues in oligodeoxyribonucleotides. J Org Chem 58:5551–5555. doi:10.1021/jo00072a050
Kneale GG (ed) (1994) DNA–protein interactions principles and protocols. Humana Press, Totowa
Lebedeva I, Stein CA (2001) Antisense oligonucleotides: promise and reality. Annu Rev Pharmacol Toxicol 41:403–419. doi:10.1146/annurev.pharmtox.41.1.403
Li H, Miller MJ (1999) Syntheses of 5′-deoxy-5′-N-hydroxylaminopyrimidine and purine nucleosides: building-blocks for novel antisense oligonucleosides with hydroxamate linkages. J Org Chem 64:9289–9293. doi:10.1021/jo991153b
Li H, Miller MJ (2000) Syntheses and binding studies of oligonucleotides containing N-hydroxycarbamate linkages: potential DNA cleaving antisense oligomers. Tetrahedron Lett 41:4323–4327. doi:10.1016/S0040-4039(00)00662-6
Manoharan M (2002) Oligonucleotide conjugates as potential antisense drugs with improved uptake, biodistribution, targeted delivery and mechanism of action. Antisense Nucleic Acid Drug Dev 12:103–128. doi:10.1089/108729002760070849
Mansoor M, Melendez AJ (2008) Advances in antisense oligonucleotide development for target identification, validation, and as novel therapeutics. Gene Regul Syst Biol 2:275–295
Maxam AM, Gilbert W (1980) Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol 65:499–553. doi:10.1016/S0076-6879(80)65059-9
Minshull J, Hunt Y (1986) The use of single-stranded DNA and RNase H to promote quantitative ‘hybrid arrest of translation’ of mRNA/DNA hybrids in reticulocyte lysate cell-free translations. Nucleic Acids Res 14:6433–6444. doi:10.1093/nar/14.16.6433
Morvan F, Porumb H, Degols G, Lefebvre I, Pompon A, Sproat BS, Rayner B, Malvy C, Lebleu B, Imbach JL (1991) Comparative evaluation of seven oligonucleotide analogs as potential antisense agents. J Med Chem 36:280–287. doi:10.1021/jm00054a013
Peyrottes S, Vasseur JJ, Imbach JL, Rayner B (1994) Synthesis, base pairing properties and nuclease resistance of oligothymidylate analogs containing methoxyphosphoramidate internucleoside linkages. Nucleosides Nucleotides 13:2135–2149. doi:10.1080/15257779408013213
Pieles U, Zurcher W, Schar M, Moser HE (1993) Matrix-assisted laser desorption ionization time-of-flight mass spectrometry: a powerful tool for the mass and sequence analysis of natural and modified oligonucleotides. Nucleic Acids Res 21:3191–3196. doi:10.1093/nar/21.14.3191
Rodger A, Norden B (eds) (1997) Circular dichroism and linear dichroism. Oxford University Press, New York
Roosenberg JMII, Lin YM, Lu Y, Miller MJ (2000) Studies and syntheses of siderophore, microbial iron chelators, and analogs as potential drug delivery agents. Curr Med Chem 7:159–197
Sanghvi YS, Cook PC (eds) (1994) Carbohydrate modifications in antisense research. ACS symposium series 580
Slater GW, Mayer P, Drouin G (1996) Migration of DNA through gels. Methods Enzymol 270:272–295. doi:10.1016/S0076-6879(96)70014-9
Stein CA (1999) Two problems in antisense biotechnology: in vitro delivery and the design of antisense experiments. Biochim Biophys Acta 1489:45–52
Uhlmann E, Peyman A (1990) Antisense oligonucleotides: a new therapeutic principle. Chem Rev 90:544–584. doi:10.1021/cr00102a001
Wickstrom E (ed) (1991) Prospects for antisense nucleic acid therapy of cancer and AIDS. Wiley-Liss, New York
Acknowledgments
We gratefully acknowledge the NIH (AI 30988) and Kimeragen, Inc. for the support of this research. H.L. is grateful to the Department of Chemistry and Biochemistry, University of Notre Dame for financial support in the form of a Reilly fellowship. C.A.F. is grateful to the Department of Chemistry and Biochemistry, University of Notre Dame for financial support in the form of a Podrasnik Fellowship. We appreciate the use of the Bioscience Core facility for the solid phase DNA synthesis, Mass Spectrometry and NMR facilities at the Department of Chemistry and Biochemistry, University of Notre Dame. We are particularly indebted to Prof. P. Huber for assistance with electrophoresis studies and helpful discussions. We also thank Ms. Maureen Metcalf for assistance with the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miller, M.J., Li, H. & Foss, C.A. Novel antisense oligonucleotides containing hydroxamate linkages: targeted iron-triggered chemical nucleases. Biometals 22, 491–510 (2009). https://doi.org/10.1007/s10534-009-9206-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-009-9206-7