Abstract
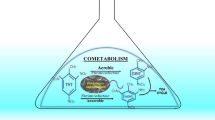
2,4-Dinitroanisole (DNAN) is a low sensitive melt-cast chemical being tested by the Military Industry as a replacement for 2,4,6-trinitrotoluene (TNT) in explosive formulations. Little is known about the fate of DNAN and its transformation products in the natural environment. Here we report aerobic biotransformation of DNAN in artificially contaminated soil microcosms. DNAN was completely transformed in 8 days in soil slurries supplemented with carbon and nitrogen sources. DNAN was completely transformed in 34 days in slurries supplemented with carbons alone and persisted in unamended microcosms. A strain of Bacillus (named 13G) that transformed DNAN by co-metabolism was isolated from the soil. HPLC and LC–MS analyses of cell-free and resting cell assays of Bacillus 13G with DNAN showed the formation of 2-amino-4-nitroanisole as the major end-product via the intermediary formation of the arylnitroso (ArNO) and arylhydroxylamino (ArNHOH) derivatives, indicating regioselective reduction of the ortho-nitro group. A series of secondary reactions involving ArNO and ArNHOH gave the corresponding azoxy- and azo-dimers. Acetylated and demethylated products were identified. Overall, this paper provides the evidence of fast DNAN transformation by the indigenous microbial populations of an amended soil with no history of contamination with explosives and a first insight into the aerobic metabolism of DNAN by the soil isolate Bacillus 13G.




Similar content being viewed by others
References
Arnett CM, Rodriguez G, Maloney SW (2009) Analysis of bacterial community diversity in anaerobic fluidized bed bioreactors treating 2,4-diaminoanisole (DNAN) and N-methyl-4-nitroaniline (MNA) using 16S rRNA gene clone libraries. Microbes Environ 24:72–75
Balakrishnan VK, Monteil-Rivera F, Halasz A, Corbeanu A, Hawari J (2004) Decomposition of the polycyclic nitramine explosive, CL-20, by Feo. Environ Sci Technol 38:6861–6866
Bhatt M, Zhao JS, Halasz A, Hawari J (2006) Biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine by novel fungi isolated from unexploded ordnance contaminated marine sediment. J Ind Microbiol Biotechnol 33:850–858
Davies PJ, Provatas A (2006) Characterization of 2,4-dinitroanisole: an ingredient for use in low sensitivity melt cast formulations. Weapons Systems Division, Defense Science and Technology Organization, Department of Defense, Commonwealth Australia
Haidour A, Ramos JL (1996) Identification of products resulting from the biological reduction of 2,4,6-trinitrotoluene, 2,4-dinitrotoluene, and 2,6-dinitrotoluene by Pseudomonas sp. Environ Sci Technol 30:2365–2370
He Z, Spain JC (1999) Comparison of the downstream pathways for degradation of nitrobenzene by Pseudomonas pseudoalcaligenes JS45 (2-aminophenol pathway) and by Comamonas sp. JS765 (catechol pathway). Arch Microbiol 171:309–316
Jain RK, Dreisbach JH, Spain JC (1994) Biodegradation of p-nitrophenol through 1,2,4-benzenetriol by an Arthrobacter sp. Appl Environ Microbiol 60:3030–3032
Ju K, Parales RE (2010) Nitroaromatic compounds, from synthesis to biodegradation. Microbiol Mol Biol Rev 74:250–272
Kim HY, Song HG (2005) Purification and characterization of NAD(P)H-dependent nitroreductase I from Klebsiella sp. C1 and enzymatic transformation of 2,4,6-trinitrotoluene. Appl Microbiol Biotechnol 68:766–773
Nishino SF, Spain JC (1995) Oxidative pathway for the biodegradation of nitrobenzene by Comamonas sp. strain JS765. Appl Environ Microbiol 61:2308–2313
Nishino SF, Paoli GC, Spain JC (2000) Aerobic degradation of dinitrotoluenes and pathway for bacterial degradation of 2,6-dinitrotoluene. Appl Environ Microbiol 66:2139–2147
Platten WE III, Bailey D, Suidan MK, Maloney SW (2010) Biological transformation pathways of 2,4-dinitro anisole and N-methyl paranitro aniline in anaerobic fluidized-bed bioreactors. Chemosphere 81:1131–1136
Schäfer A, Harms H, Zehnder AJ (1996) Biodegradation of 4-nitroanisole by two Rhodococcus spp. Biodegradation 7:249–255
Spain JC (1995) Biodegradation of nitroaromatic compounds. Annu Rev Microbiol 49:523–555
U.S. Department of Health and Human Services (1995) Toxicological profile for dinitrophenols. US Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta
Vasilyeva GK, Oh B-T, Shea PJ, Drijber RA, Kreslavcki VD, Minard R, Bollag J-M (2000) Aerobic TNT reduction via 2-hydroxylamino-4,6-dinitrotoluene by Pseudomonas aeruginosa strain MX isolated from munitions-contaminated soil. Biorem J 4:111–124
Wang CY, Zheng D, Highes JB (2000) Stability of hydroxylamino- and amino intermediates from reduction of 2,4,6-trinitrotoluene, 2,4-dinitrotoluene and 2,6-dinitrotoluene. Biotechnol Lett 22:15–19
Acknowledgments
We acknowledge Defence Research Development Canada, Department of National Defence, for funding of this project. We thank L. Paquet, A. Corriveau, C. Beaulieu and S. Deschamps for the analytic support. We would like to thank Dr. Arthur Provatas from Defence Science and Technology Organisation (DSTO), Australia, for helpful discussions and for providing us with literature reports on DNAN.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perreault, N.N., Manno, D., Halasz, A. et al. Aerobic biotransformation of 2,4-dinitroanisole in soil and soil Bacillus sp.. Biodegradation 23, 287–295 (2012). https://doi.org/10.1007/s10532-011-9508-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-011-9508-7