Abstract
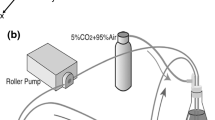
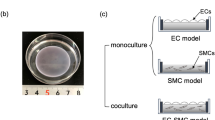
To evaluate interactions between human endothelial cells (ECs) and smooth muscle cells (SMCs) for the development of tissue-engineered vessels, we examined the adhesion and key cell properties of human ECs grown on quiescent human aortic SMCs. ECs attached to SMCs spread more slowly than ECs attached to fibronectin surfaces, and ECs aligned along the direction of the SMCs. ECs attached firmly and less than 5% of the cells were removed by shear stresses as high as 300 dyn cm−2. Unlike porcine SMCs and co-cultures, human SMCs or co-cultures do not contract under flow, and the human ECs and SMCs in co-culture align toward the direction of flow. A confluent endothelium could be maintained in co-culture for over 30 days, and some of the ECs reoriented perpendicular to the SMCs after 9 days in static culture. Surface tissue factor levels in ECs and SMCs were less in co-culture than in monoculture. Co-culture induced an increase in calponin expression in SMCs. These findings show that human co-cultures can be maintained for long culture periods, where the endothelium remains confluent and responds to long-term exposure to flow, and EC–SMC interactions lead to an increase in SMC differentiation and an EC surface that is less thrombotic.










Similar content being viewed by others
References
Ainslie K. M., Garanich J. S., Dull R. O., J. M. Tarbell Vascular smooth muscle cell glycocalyx influences shear stress-mediated contractile response. J. Appl. Physiol. 98:242–249, 2005
Bhat V. D., G. A. Truskey, W. M. Reichert. Fibronectin and avidin–biotin as a heterogeneous ligand system for enhanced endothelial cell adhesion. J. Biomed. Mater. Res. 41:377–385, 1998
Brown D. J., E. M. Rzucidlo, B. L. Merenick, R. J. Wagner, K. A. Martin, R. J. Powell. Endothelial cell activation of the smooth muscle cell phosphoinositide 3-kinase/Akt pathway promotes differentiation. J. Vasc. Surg. 41:509–516, 2005
Brown, M., C. S. Wallace, and G. A. Truskey. Vascular Endothelium. In: Wiley Encyclopedia of Biomedical Engineering, edited by M. Akay. Indianapolis, IN: Wiley Publishing Inc., 2006
Chan B. P., W. Liu, B. Klitzman, W. M. Reichert, G. A. Truskey. In vivo performance of dual ligand augmented endothelialized expanded polytetrafluoroethylene vascular grafts. J. Biomed. Mater. Res. B Appl. Biomater. 72:52–63, 2005
Chiu J. J., L. J. Chen, C. N. Chen, P. L. Lee, C. I. Lee. A model for studying the effect of shear stress on interactions between vascular endothelial cells and smooth muscle cells. J. Biomech. 37:531–539, 2004
Civelek M., K. Ainslie, J. S. Garanich, J. M. Tarbell. Smooth muscle cells contract in response to fluid flow via a Ca2+-independent signaling mechanism. J. Appl. Physiol. 93:1907–1917, 2002
Davies P. F., G. A. Truskey, H. B. Warren, S. E. O’Connor, B. H. Eisenhaure. Metabolic cooperation between vascular endothelial cells and smooth muscle cells in co-culture: Changes in low density lipoprotein metabolism. J. Cell. Biol. 101:871–879, 1985
Fillinger M. F., L. N. Sampson, J. L. Cronenwett, R. J. Powell, R. J. Wagner. Coculture of endothelial cells and smooth muscle cells in bilayer and conditioned media models. J. Surg. Res. 67:169–178, 1997
George E. L., E. N. Georges-Labouesse, R. S. Patel-King, H. Rayburn, R. O. Hynes. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 119:1079–1091, 1993
Ghrib F., A. C. Brisset, D. Dupouy, A. D. Terrisse, C. Navarro, Y. Cadroy, B. Boneu, P. Sie. The expression of tissue factor and tissue factor pathway inhibitor in aortic smooth muscle cells is up-regulated in synthetic compared to contractile phenotype. Thromb. Haemost. 87:1051–1056, 2002
Heydarkhan-Hagvall S., S. Chien, S. Nelander, Y. C. Li, S. Yuan, J. Lao, J. H. Haga, I. Lian, P. Nguyen, B. Risberg, Y. S. Li. DNA microarray study on gene expression profiles in co-cultured endothelial and smooth muscle cells in response to 4- and 24-h shear stress. Mol. Cell. Biochem. 281:1–15, 2006
Heydarkhan-Hagvall S., G. Helenius, B. R. Johansson, J. Y. Li, E. Mattsson, B. Risberg. Co-culture of endothelial cells and smooth muscle cells affects gene expression of angiogenic factors. J. Cell. Biochem. 89:1250–1259, 2003
Jude B., C. Zawadzki, S. Susen, D. Corseaux. Relevance of tissue factor in cardiovascular disease. Arch. Mal. Coeur. Vaiss. 98:667–671, 2005
Korff T., S. Kimmina, G. Martiny-Baron, H. G. Augustin. Blood vessel maturation in a 3-dimensional spheroidal coculture model: Direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J. 15:447–457, 2001
Lavender M. D., Z. Pang, C. S. Wallace, L. E. Niklason, G. A. Truskey. A system for the direct co-culture of endothelium on smooth muscle cells. Biomaterials 26:4642–4653, 2005
Lewis J. C., N. L. Jones, M. I. Hermanns, O. Rohrig, C. L. Klein, C. J. Kirkpatrick. Tissue factor expression during coculture of endothelial cells and monocytes. Exp. Mol. Pathol. 62:207–218, 1995
Lo C. M., H. B. Wang, M. Dembo, Y. L. Wang. Cell movement is guided by the rigidity of the substrate. Biophys. J. 79:144–152, 2000
Mitchell S. L., L. E. Niklason. Requirements for growing tissue-engineered vascular grafts. Cardiovasc. Pathol. 12:59–64, 2003
Nackman, G. B., F. R. Bech, M. F. Fillinger, R. J. Wagner, and J. L. Cronenwett. Endothelial cells modulate smooth muscle cell morphology by inhibition of transforming growth factor-beta 1 activation. Surgery 120:418–425; discussion 425–416, 1996
Niklason L. E., J. Gao, W. M. Abbott, K. K. Hirschi, S. Houser, R. Marini, R. Langer. Functional arteries grown in vitro. Science 284:489–493, 1999
Niwa K., T. Kado, J. Sakai, T. Karino. The effects of a shear flow on the uptake of LDL and acetylated LDL by an EC monoculture and an EC–SMC coculture. Ann. Biomed. Eng. 32:537–543, 2004
Pang Z., D. A. Antonetti, J. M. Tarbell. Shear stress regulates HUVEC hydraulic conductivity by occludin phosphorylation. Ann. Biomed. Eng. 33:1536–1545, 2005
Powell R. J., J. Bhargava, M. D. Basson, B. E. Sumpio. Coculture conditions alter endothelial modulation of TGF-beta 1 activation and smooth muscle growth morphology. Am. J. Physiol. 274:H642–H649, 1998
Powell R. J., J. L. Cronenwett, M. F. Fillinger, R. J. Wagner, L. N. Sampson. Endothelial cell modulation of smooth muscle cell morphology and organizational growth pattern. Ann. Vasc. Surg. 10:4–10, 1996
Rainger G. E., P. Stone, C. M. Morland, G. B. Nash. A novel system for investigating the ability of smooth muscle cells and fibroblasts to regulate adhesion of flowing leukocytes to endothelial cells. J. Immunol. Methods 255:73–82, 2001
RayChaudhury A., M. Elkins, D. Kozien, M. T. Nakada. Regulation of PECAM-1 in endothelial cells during cell growth and migration. Exp. Biol. Med. (Maywood) 226:686–691, 2001
Sato Y. Activation of latent TGF-beta at the vascular wall-roles of endothelial cells and mural pericytes or smooth muscle cells. J. Atheroscler. Thromb. 2:24–29, 1995
Sato Y., F. Okada, M. Abe, T. Seguchi, M. Kuwano, S. Sato, A. Furuya, N. Hanai, T. Tamaoki. The mechanism for the activation of latent TGF-beta during co-culture of endothelial cells and smooth muscle cells: Cell-type specific targeting of latent TGF-beta to smooth muscle cells. J. Cell. Biol. 123:1249–1254, 1993
Sato Y., R. Tsuboi, R. Lyons, H. Moses, D. B. Rifkin. Characterization of the activation of latent TGF-beta by co-cultures of endothelial cells and pericytes or smooth muscle cells: a self-regulating system. J. Cell. Biol. 111:757–763, 1990
Truskey G. A., J. S. Pirone. The effect of fluid shear stress upon cell adhesion to fibronectin-treated surfaces. J. Biomed. Mater. Res. 24:1333–1353, 1990
van Buul-Wortelboer M. F., H. J. Brinkman, K. P. Dingemans, P. G. de Groot, W. G. van Aken, J. A. van Mourik. Reconstitution of the vascular wall in vitro. A novel model to study interactions between endothelial and smooth muscle cells. Exp. Cell. Res. 162:151–158, 1986
Wada Y., A. Sugiyama, T. Kohro, M. Kobayashi, M. Takeya, M. Naito, T. Kodama. In vitro model of atherosclerosis using coculture of arterial wall cells and macrophage. Yonsei Med. J. 41:740–755, 2000
Yang J. T., H. Rayburn, R. O. Hynes. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development 119:1093–1105, 1993
Zhang J. C., Q. Ruan, L. Paucz, A. Fabry, B. R. Binder, J. Wojta. Stimulation of tissue factor expression in human microvascular and macrovascular endothelial cells by cultured vascular smooth muscle cells in vitro. J. Vasc. Res. 36:126–132, 1999
Ziegler T., R. W. Alexander, R. M. Nerem. An endothelial cell-smooth muscle cell co-culture model for use in the investigation of flow effects on vascular biology. Ann. Biomed. Eng. 23:216–225, 1995
Acknowledgments
The authors would like to thank Zhengyu Pang and Jeffrey LaMack for their technical advice. This work was supported by NIH Grant R21HL 72189.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wallace, C.S., Champion, J.C. & Truskey, G.A. Adhesion and Function of Human Endothelial Cells Co-cultured on Smooth Muscle Cells. Ann Biomed Eng 35, 375–386 (2007). https://doi.org/10.1007/s10439-006-9230-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-006-9230-5