Abstract
Herpesvirus has the potential to infect a wide variety of animal species. In cetaceans, Alpha- and/or Gammaherpesvirinae have been identified in eight families of odontocetes, and one family of mysticetes. In May 2022, an adult humpback whale (Megaptera novaeangliae) was found stranded in Valencia, Spain. The whale was emaciated, in poor body condition, with multiple lacerations on the dorsal fin and a high number of epibionts of the Cyamidae family, known as whale lice. The individual had been previously released from a ghost net entanglement 5 days before becoming stranded. In a closer examination, various skin lesions were observed, including chronic, proliferative, and erosive dermatitis and a large ulcer extending to the deep dermis. As part of the infectious disease surveillance programme, molecular testing was performed on skin samples for herpesvirus, cetacean morbillivirus, and poxvirus. A positive result for herpesvirus was obtained from one of the skin lesions. The sequence was found to belong to the Alphaherpesvirinae subfamily, and it was closely related to alphaherpesvirus sequences from a fin whale (Balaenoptera physalus) and a humpback whale. Cetacean morbillivirus and poxvirus testing was negative. To the authors’ knowledge, this is the first report of herpesvirus in a humpback whale from the Mediterranean Sea. Reports on herpesvirus detection or infection in humpback whales (only species within the genus Megaptera) are scarce. In consequence, future virological assessments of humpback whales should include testing for herpesvirus.
Similar content being viewed by others
Introduction
According to the International Committee on Taxonomy of Viruses, the family Orthoherpesviridae is a linear, double-stranded DNA (125–290 kbp) virus family (Davison et al. 2009) which is subdivided into 3 subfamilies: Alphaherpesvirinae, Betaherpesvirinae, and Gammaherpesvirinae (International Committee on Taxonomy of Viruses—Family: Orthoherpesviridae, https://ictv.global/report/chapter/orthoherpesviridae/orthoherpesviridae, accesed in September 1st 2023).
A wide range of animal species can be infected by herpesvirus (HV), including mammals, birds, reptiles, fish, amphibians, and bivalves (Davison et al. 2009). Alpha- and/or Gammaherpesvirinae have been previously identified in eight odontocete families, including marine and riverine cetaceans: Delphinidae, Kogiidae, Ziphiidae, Physeteridae, Monodontidae, Phocoenidae, Iniidae, and Pontoporiidae (Smolarek et al. 2006; Arbelo et al. 2010; Miyoshi et al. 2011; Bellehumeur et al. 2015; van Beurden et al. 2015; Seade et al 2017, Sacristán et al. 2019, Exposto Novoselecki et al. 2021). Regarding mysticetes, HV has been detected exclusively in the Balaenopteridae family: alphaherpesvirus (AHV) was detected in the skin and penile mucosa of a fin whale (Balaenoptera physalus), and gammaherpesvirus (GHV) was amplified from the skin, muscle, and central nervous system of a common minke whale (Balaenoptera acutorostrata) (Melero et al. 2015), as well as from the central nervous system of another common minke whale (Davison et al. 2021). Recently, an infection by an AHV has been described in the lung of a humpback whale (Megaptera novaeangliae) (Sacristán et al. 2024).
A wide variety of lesions have been associated with herpesvirus infections in cetaceans. Alphaherpesviruses have been associated to skin lesions (Manire et al. 2006; Smolarek et al. 2006; Sacristán et al. 2019; Vargas-Castro et al. 2021), systemic infections (Blanchard et al. 2001; Arbelo et al. 2010, 2012; Soto et al. 2012; Vargas-Castro et al. 2021), and encephalitis (Esperón et al. 2008; Sierra et al. 2014; Sierra et al. 2022). Additionally, alphaherpesviruses have also been found in genital lesions (Bellehumeur et al. 2015). On the other hand, Gammaherpesviruses have been detected in both generalized infections (Vargas-Castro et al. 2020) and central nervous system infections (Melero et al. 2015; Vargas-Castro et al. 2020, 2021; Sierra et al. 2022; Giorda et al. 2022). Nevertheless, gammaherpesviruses have primarily been associated with cutaneous (Sacristán et al. 2019; Vargas-Castro et al. 2021) and mucosal lesions, encompassing genital (Saliki et al. 2006; Smolarek et al. 2006; van Elk et al. 2009; Sierra et al. 2015; Seade et al. 2017; Vargas-Castro et al. 2020; Vargas-Castro et al. 2021) and upper digestive mucosa (Ewing et al. 2020; Vargas-Castro et al. 2021; Vargas-Castro et al. 2023). Herpesvirus infections in cetaceans have been detected in individuals lacking clinical signs and lesions as well (Bellière et al. 2010; Vargas-Castro et al. 2020, 2021; Felipe-Jiménez et al. 2021; Exposto Novoselecki et al. 2021). Additionally, HV can lead to immunosuppression in cetaceans (Arbelo et al. 2010).
In recent years, there has been a notable rise in the number of humpback whale sightings in the Mediterranean Sea. The presence of these species in this location is believed to be linked to feeding reasons (Espada Ruíz et al. 2018).
This study reports the molecular detection of a novel AHV from a skin lesion observed in a humpback whale, addressing new insights into the scarce knowledge available for herpesvirus in this species. To our knowledge, this finding represents the first documented case of HV infection in a humpback whale within the Mediterranean Sea. Furthermore, it constitutes the first characterization of a cutaneous lesion associated with HV observed in this species.
Case presentation
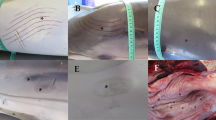
On 26th May 2022, an adult humpback whale (Megaptera novaeangliae) of approximately 25 tons and 12 m in length stranded alive in Tavernes de la Valldigna, Valencia, Spain (39° 4′ 18″ N 0° 16′ 4″ W). Five days before stranding, this individual was released from a ghost net entanglement in the Balearic Island of Mallorca (Spain), approximately 320 km away. The animal was extremely weak, emaciated, and unable to swim (Fig. 1A and B), and died 3 h after beach stranding. Due to logistical difficulties, a complete necropsy could not be performed, and the sex of the animal could not be determined. However, in the post-mortem external evaluation, it was observed that approximately 30% of the dorsal body area was completely covered by epibionts of the Cyamidae family (Fig. 1A–D). Additionally, the animal had several linear lacerations on the fluke, dorsal, and pectoral fins (Fig. 2, lesion showed in Fig. 2C is considered lesion 1). A few of these lesions were open wounds extending to the dermis, and the adjacent epidermis was pale grey to white, irregularly raised, and firm, indicating epithelial proliferation and repair. The exposed surface was overlaid by necrotic debris and pale yellow, viscous material consistent with pus. The lesions were consistent with entanglement injuries.
Macroscopic details providing insights into the overall health status of the stranded humpback whale. A Aerial view of the animal. The individual floated but was unable to swim. The orange colour corresponds to the massive infestation of whale lice belonging to the Cyamidae family. B Caudal view of the emaciated animal, revealing atrophy of epaxial musculature and prominent bone structures. C Massive infestation of whale lice belonging to the Cyamidae family around the head and rostrum of the stranded humpback whale. D Greater detail of whale lice infestation around the eye
In addition, throughout the skin, there were well demarcated, variably sized, pale grey foci, with depressed centers or raised, bullous edges (Fig. 3). In the right flank, there were multifocal, irregular, whitish, or pale grey patches, with depressed centers consistent with erosions, surrounded by a pale-grey rim (lesion 2, Fig. 3A and D). Also in the right flank, there were multiple, 1–3 cm in diameter, firm, raised nodules (lesion 3, Fig. 3B and D). Lastly, in some areas, the epidermis was irregularly raised, white and grey, and soft, with central, depressed, dark grey areas of erosion (lesion 4, Fig. 3C and D).
Degenerative and proliferative dermatitis observed in the stranded humpback whale. A Skin lesion 2. Well and poorly demarcated areas with central erosion surrounded by pale grey to white halo in the right flank. B Skin lesion 3. Multiple nodules, measuring between 1 and 3 cm and with a firm consistency, slightly raised in the right flank. C Skin lesion 4. Area of necrotizing dermatitis with multifocal sloughed and retained epidermis and erosions. D Area on the right flank in which the three lesions illustrated in A, B, and C can be observed collectively. The upper portion of the image displays the lesion 4, while the middle section shows the lesion 2. The lesion 3 can be seen in the lower part of the image
During the post-mortem examination, the following tissues were sampled: normal skin, skin lesions 1–4 (Fig. 2C and Fig. 3A–C), muscle, and blubber. Two sets of tissue samples were collected: the first set was preserved in 10% neutral buffered formalin for routine histopathology; the second set was stored at −80 °C for molecular analysis.
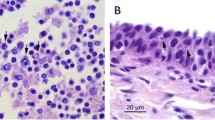
Histologic examination of skin lesions revealed a continuum of chronic, proliferative dermatitis with secondary bacterial infection and pustules. Lesions in the right flank consisted of over 70 layers of epithelium, with piled and slightly disorganized epithelial cells in the basal and middle layers (acanthosis). Basal layers were tightly piled and disorganized along a tortuous basement membrane, with deep and anastomosing rete pegs. The stratum spinosum contained numerous, slightly swollen squamous cells with pale eosinophilic or colourless cytoplasm. Multifocally, superficial layers were variably absent (eroded). Additionally, in the superficial dermis between rete pegs, there were mild to moderate, perivascular infiltrates of neutrophils, macrophages, and lymphocytes (Fig. 4A). The skin from lesion 4, in addition to the acanthosis and dermal inflammation similar to Fig. 4A, contained severe intracellular edema and degeneration of the stratum spinosum, with many, multifocal, up to 250 µm, colourless spaces (pustules) filled with neutrophils, protein fluid, cell debris, and small bacterial colonies (Fig. 4B).
Main histopathological findings. A Epidermal hyperplasia, with basal cells piling up and being disorganized along the basement membrane of tortuous and anastomosing rete pegs. Basal and middle layers with moderate, multifocal intracellular edema and keratinocyte degeneration. Additionally, there is mild to moderate mixed infiltration of neutrophils, macrophages, and lymphocytes in perivascular areas. B Intracellular edema in the stratum spinosum and degeneration, with multiple, scattered pustules contained neutrophils, protein fluid, cellular debris, and small bacterial colonies. C Full-thickness epidermal defect that extends into the dermis. D The dermis below the ulcer is replaced by granulation tissue infiltrated by numerous neutrophils and eosinophils
The skin in lesion 1 contained a large ulceration that extended into the deep dermis (Fig. 4C) and contained numerous viable and degenerated neutrophils, nuclear and cytoplasmic debris, fibrin, and haemorrhage. The adjacent dermis was extensively replaced by exuberant granulation tissue (consistent with a keloid) composed of interlacing, haphazardly arranged, streams of hypertrophied fibroblasts, parallel arranged in some areas, separated by small amounts of loose collagenous matrix, clear spaces and numerous perpendicularly aligned capillaries with hypertrophic endothelium. Additionally, the matrix contained numerous neutrophils, lymphocytes, macrophages, and fewer plasma cells. The epidermis overlaying the granulation tissue adjacent to the ulcer was markedly hyperplastic (Fig. 4D).
The tissue samples were evaluated for the presence of cetacean morbillivirus (CeMV), herpesvirus, and poxvirus infection. Standard precautions were taken during all laboratory procedures to avoid cross-contamination of samples. All samples were homogenized using stainless steel 4.8-mm beads (Next Advance, New York, USA) after being added to phosphate-buffered saline (PBS) at a 1:10 proportion. RNA and DNA were extracted using the High Pure Viral Nucleic Acid Kit (Roche Diagnostics), based on the manufacturer’s instructions.
For the molecular diagnosis of HV, a previously described pan-herpesvirus nested PCR targeting a fragment of the DNA polymerase (DNApol) gene (VanDevanter et al. 1996) was performed. This approach is very useful, not only for detecting new sequences of HV (VanDevanter et al. 1996), but also because cetacean HV strains are usually classified according to the sequence of a part of a locus of their DNApol (Noguchi et al. 2013).
Appropriate non-template negative controls (nuclease-free water) for both extraction and PCR as well as extraction-positive and PCR-positive controls were included.
Only one of the skin lesions (lesion 2, Fig. 3A) yielded a positive result. All healthy skin samples were negative. The agarose band was purified using the QIAquick® Gel Extraction Kit (Qiagen, Hilden, Germany), and 212-bp amplicon was completely sequenced by Sanger sequencing. The nucleotide sequence confirmed the presence of herpesvirus in the specified sample and was deposited in GenBank under accession number OQ561785.
A nucleotide Maximum likelihood phylogenetic tree was constructed based on Tamura 3-parameter model using MEGA 11 (Tamura et al. 2021). A discrete Gamma distribution was used to model evolutionary rate differences among sites with five categories and the rate variation model allowed for some sites to be evolutionarily invariable. In order to produce a reliable phylogenetic tree, the accuracy of sequence alignment was verified, since the average amino acid p-distance (1-amino acid identity), was 0.64, and therefore, was lower than the acceptance threshold of < 0.8 (Thompson et al. 1999; Ogden and Rosenberg 2006). Bootstrap analysis with 2000 replicates was performed to test the reliability of the tree.
For this phylogenetic study, one human herpesvirus sequence, 25 odontocete herpesvirus sequences, and four mysticete herpesvirus sequences detected in different countries worldwide since 1999 were included, in addition to the sequence described in this study.
The phylogenetic analysis revealed that the sequence OQ561785 is an AHV (Fig. 5) and clusters together with AHV detected in a fin whale from Spain (KP995686) and in a humpback whale from Brazil (OQ561785). Accordingly, this novel sequence showed the highest homology to GenBank sequences KP995686 (nucleotide identity, 90.15%; amino acid identity, 88.37%) and OQ561785 (nucleotide identity, 89.39%; amino acid identity, 86.05%).
Maximum-likelihood phylogram of herpesviruses based on partial DNApol gene sequence. The nucleotide sequence reported in the present study is identified only with the bolded accession number and a square, while other herpesvirus sequences are labeled with accession number, host species, herpesvirus subfamily, and country and year of origin
The tissue samples were tested for CeMV using a reverse transcription-PCR method that targets the fusion protein gene and is based on the Universal Probe Library platform (Rubio-Guerri et al. 2013). Ultrapure water was used as a negative control, while striped dolphin CeMV-positive lung RNA was used as a positive control. All samples were negative. Additionally, a conventional PCR (Bracht et al. 2006) was used to test DNA extracts of the four skin lesions for Poxvirus, using ultrapure water as a negative control and a striped dolphin poxvirus-positive skin DNA as a positive control. However, all samples yielded negative results.
Discussion
In the last few years, HV infections have been frequently reported in odontocetes (Smolarek et al. 2006; Arbelo et al. 2010; Miyoshi et al. 2011; Bellehumeur et al. 2015; van Beurden et al. 2015; Sacristán et al. 2019). In contrast, reports of HV in mysticetes are relatively scarce (Melero et al. 2015; Davison et al. 2021, Sacristán et al. 2024). The limited opportunities for sampling stranded mysticetes, in comparison to odontocetes (Nemiroff et al. 2010; Coombs et al. 2019), might offer an explanation for the observed discrepancy, thus making it challenging to determine the causes of mortality and morbidity in large whales (Groch et al. 2018). This means that each stranded mysticete individual can provide a significant amount of valuable information. For this reason, it is crucial to conduct health surveillance to monitor for pathogens that can impact these animals.
Previous research on the presence of HV in humpback whales is limited. Miyoshi et al. (2011) conducted an earlier study on this topic, which, however, did not yield any HV-positive results (Miyoshi et al. 2011). Recently, a study conducted on cetaceans stranded in Brazil surveyed HV presence in 18 humpback whales, with only one individual testing positive (Sacristán et al. 2024). This was the first description of a HV in this baleen species (Sacristán et al. 2024). In the present study, to the best of the authors’ knowledge, we report the first molecular detection of HV in a humpback whale from the Mediterranean Sea, addressing new insights into the scarce knowledge available for herpesvirus in this species. This discovery supports the hypothesis that humpback whales may serve as a new potential host (Sacristán et al. 2024), and consequently, it is recommended to include herpesvirus in the virological assessment of these species. The novel herpesvirus sequence described in this study from a humpback whale was an AHV and displayed the highest degree of identity with AHV sequences characterized from both a fin whale from Spain and a humpback whale from Brazil, as observed in the phylogenetic analysis. This finding is in line with earlier observations regarding HV in marine mammals, which suggest that herpesvirus phylogenetic branching resembles that of its hosts (Maness et al. 2011; Melero et al. 2014, 2015; Exposto Novoselecki et al. 2021), because a co-speciation with host could have occurred (McGeoch et al. 2006).
The area of skin with epidermal erosion surrounded by a sharply delimited pale grey halo (lesion 2, Fig. 3A) tested positive for HV. Previous studies have described skin lesions in cetaceans potentially associated with herpesviruses, which exhibit a similar pale or whitish discoloration, as well as the presence of a hypopigmented halo (Hart et al. 2012; Toms et al. 2020). Histopathological analysis of lesions 3 and 4 revealed a chronic, proliferative dermatitis (Fig. 4C and D). Acanthosis, intracellular edema, and degeneration, together with mixed inflammation were observed in these lesions, and these features have been associated with herpesviral infection (Manire et al. 2006; Vargas-Castro et al. 2021). However, caution must be taken in interpreting these findings since hyperplasia and inflammation may also be associated with whale lice infestation (Lehnert et al. 2021). Intranuclear inclusions were not observed in any of the lesions; however, this does not rule out a possible association between the epithelial proliferation and/or degeneration and the pathological effects of the virus, since intranuclear inclusions are only reported in certain stages of viremia (Hart et al 2012) and are inconsistently observed in skin lesions in other cetaceans (Manire et al 2006; Hart et al 2012; Bellehumeur et al 2015).
Unfortunately, a complete necropsy was not possible, and we cannot rule out if other organs were affected as well. We strongly recommend that future studies analyze as many samples as possible to obtain a better understanding of the stranding event.
In addition, this individual exhibited several lacerations on the dorsal, caudal, and pectoral fins. Considering the individual was disentangled from a ghost net 5 days before it stranded and given that the location of the injuries corresponded to the entanglement points, we attribute these lacerations as a result of the entanglement event. Apart from the aforementioned injuries, other processes have been linked to such events, including weakening, starvation, and chronic stress, which increase susceptibility to opportunistic infections, as previously suggested (Cassoff et al. 2011). This corresponds to the case described in our study, wherein the animal was emaciated and weakened, and exhibited a cutaneous herpesviral infection, superficial bacterial infection, and massive infestation by whale lice. The simultaneous occurrence of these agents emphasizes the frequent incidence of concurrent infections linked to herpesvirus presence in cetaceans (Casalone et al. 2014; Sierra et al. 2014; Bento et al. 2019; Sierra et al. 2020; Vargas-Castro et al. 2021; Sierra et al. 2022), which may indicate an underlying immunosuppression, that can be exacerbated by HV infection, as this agent is also considered to be immunosuppressive (Arbelo et al. 2010).
High numbers of Cyamidae whale lice have been associated to mobility restriction of whales with chronic entanglement (Cassoff et al 2011) and vertebral injuries (Groch et al 2012), and have been used as an indicator of poor condition of humpback whales (Osmond and Kaufman 1998) and North Atlantic right whales (Eubalaena glacialis) (Knowlton and Kraus 2001), which is consistent with this study, since the animal presented a high density of whale lice.
To the best of authors’ knowledge, this study provides a first description of cutaneous AHV infection in a humpback whale. In addition, the animal had multifocal chronic, degenerative, and proliferative dermatitis in some areas concomitant with HV positivity. Given the lack of specific information regarding HV-associated pathology in mysticetes, this novel finding represents a significant contribution to our understanding of herpesvirus infections in these animals and emphasizes the need for further studies to determine the prevalence, transmission, and clinical significance, since the potential impact of HV infection on humpback whale populations is still unclear. Moreover, this study underscores the significance of conducting sanitary surveillance investigations on stranded cetaceans, especially in mysticetes, for the pathogens that may impact their health, as previously suggested (Melero et al. 2015). To enhance the probability of detecting HV-positive samples, it is recommended that future studies involving mysticetes systematically collect samples from as many organs as possible, as a greater number of analyzed samples per animal have been associated with a higher likelihood of discovering HV-positive individuals (Sierra et al. 2022).
Conclusion
In conclusion, a novel AHV has been identified from a humpback whale, which exhibited a chronic, proliferative, and erosive dermatitis. Phylogenetic analysis revealed the closest relationship to an AHV isolated from other mysticete species. To our knowledge, this is the first report of herpesviruses in a humpback whale from the Mediterranean Sea.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- HV:
-
Herpesvirus
- AHV:
-
Alphaherpesvirus
- GHV:
-
Gammaherpesvirus
- CeMV:
-
Cetacean morbillivirus
- DNApol:
-
DNA polymerase
References
Arbelo M, Bellière EN, Sierra E, Sacchinni S, Esperón F, Andrada M, Rivero M, Diaz-Delgado J, Fernández A (2012) Herpes virus infection associated with interstitial nephritis in a beaked whale (Mesoplodon densirostris). BMC Vet Res 8(1):243
Arbelo M, Sierra E, Esperón F, Watanabe TT, Bellière EN, Espinosa de los Monteros A, Fernández A. (2010) Herpesvirus infection with severe lymphoid necrosis affecting a beaked whale stranded in the Canary Islands. Dis Aquat Organ 89(3):261–264
Bellehumeur C, Lair S, Romero CH, Provost C, Nielsen O, Gagnon CA (2015) Identification of a novel herpesvirus associated with a penile proliferative lesion in a beluga (Delphinapterus leucas). J Wildl Dis 51(1):244–249, 246
Bellière EN, Esperón F, Arbelo M, Muñoz MJ, Fernández A, Sánchez-Vizcaíno JM (2010) Presence of herpesvirus in striped dolphins stranded during the cetacean morbillivirus epizootic along the Mediterranean Spanish coast in 2007. Arch Virol 155(8):1307–1311
Bento MC, Canha R, Eira C, Vingada J, Nicolau L, Ferreira M, Domingo M, Tavares L, Duarte A (2019) Herpesvirus infection in marine mammals: a retrospective molecular survey of stranded cetaceans in the Portuguese coastline. Infect Genet Evol 67:222–233
Blanchard TW, Santiago NT, Lipscomb TP, Garber RL, McFee WE, Knowles S (2001) Two novel alphaherpesviruses associated with fatal disseminated infections in Altlantic bottlenose dolphins. J Wildl Dis 37(2):297–305
Bracht AJ, Brudek RL, Ewing RY, Manire CA, Burek KA, Rosa C, Beckmen KB, Maruniak JE, Romero CH (2006) Genetic identification of novel poxviruses of cetaceans and pinnipeds. Adv Virol 151(3):423–438
Casalone C, Mazzariol S, Pautasso A, Di Guardo G, Di Nocera F, Lucifora G, Ligios C, Franco A, Fichi G, Cocumelli C et al (2014) Cetacean strandings in Italy: an unusual mortality event along the Tyrrhenian Sea coast in 2013. Dis Aquat Organ 109(1):81–86
Cassoff RM, Moore KM, McLellan WA, Barco SG, Rotstein DS, Moore MJ (2011) Lethal entanglement in baleen whales. Dis Aquat Org 96(3):175–185
Coombs EJ, Deaville R, Sabin RC, Allan L, O’Connell M, Berrow S, Smith B, Brownlow A, Doeschate MT, Penrose R (2019) What can cetacean stranding records tell us? A study of UK and Irish cetacean diversity over the past 100 years. Mar Mamm Sci 35(4):1527–1555
Davison AJ, Eberle R, Ehlers B, Hayward GS, McGeoch DJ, Minson AC, Pellett PE, Roizman B, Studdert MJ, Thiry E (2009) The order Herpesvirales. Arch Virol 154(1):171–177
Davison NJ, Dagleish MP, Ten Doeschate M, Muchowski J, Perrett LL, Rocchi M, Whatmore AM, Brownlow A (2021) Meningoencephalitis in a common minke whale Balaenoptera acutorostrata associated with Brucella pinnipedialis and gamma-herpesvirus infection. Dis Aquat Organ 144:231–235
Espada Ruíz R, Olaya-Ponzone L, García-Gómez JC (2018) Humpback whale in the bay of Algeciras and a mini-review of this species in the Mediterranean. Reg Stud Mar Sci 24:156–164
Esperón F, Fernández A, Sánchez-Vizcaíno JM (2008) Herpes simplex-like infection in a bottlenose dolphin stranded in the Canary Islands. Dis Aquat Organ 81(1):73–76
Exposto Novoselecki H, Catão-Dias JL, Ewbank AC, Navas-Suárez PE, Duarte-Benvenuto A, Lial HC, Costa Silva S, Sánchez-Sarmiento AM, Gravena W, Ferreira da Silva VM, Carvalho VL, Marmontel M, Bertozzi CP, Lanes Ribeiro V, del Rio do Valle R, Marigo J, das Neves CG, Esperón F, Sacristán C. (2021) Highly divergent herpesviruses in threatened river dolphins from Brazil. Sci Rep 11:24528
Ewing RY, Rotstein DS, McLellan WA, Costidis AM, Lovewell G, Schaefer AM, Romero CH, Bossart GD (2020) Macroscopic and histopathologic findings from a mass stranding of rough-toothed dolphins (Steno bredanensis) in 2005 on Marathon Key, Florida, USA. Front Vet Sci 7(572)
Felipe-Jiménez I, Fernández A, Andrada M, Arbelo M, Segura-Göthlin S, Colom-Rivero A, Sierra E (2021) Contribution to herpesvirus surveillance in beaked whales stranded in the Canary Islands. Animals 11(7):1923
Giorda F, Crociara P, Iulini B, Gazzuola P, Favole A, Goria M, Serracca L, Dondo A, Crescio MI, Audino T et al (2022) Neuropathological characterization of dolphin morbillivirus infection in cetaceans stranded in Italy. Animals 12(4):452
Groch KR, Marcondes MC, Colosio AC, Catão-Dias JL (2012) Skeletal abnormalities in humpback whales Megaptera novaeangliae stranded in the Brazilian breeding ground. Dis Aquat Organ 101(2):145–58
Groch KR, Díaz-Delgado J, Marcondes MC, Colosio AC, Santos-Neto EB, Carvalho VL, Boos GS, Oliveira de Meirelles AC, Ramos HGdC, Guimarães JP (2018) Pathology and causes of death in stranded humpback whales (Megaptera novaeangliae) from Brazil. PLoS ONE 13(5):e0194872
Hart LB, Rotstein DS, Wells RS, Allen J, Barleycorn A, Balmer BC, Lane SM, Speakman T, Zolman ES, Stolen M et al (2012) Skin lesions on common bottlenose dolphins (Tursiops truncatus) from three sites in the Northwest Atlantic, USA. PLoS One 7(3):e33081
International committee on taxonomy of viruses - family: Orthoherpesviridae. https://ictv.global/report/chapter/orthoherpesviridae/orthoherpesviridae, accesed in 1 Sept 2023
Knowlton AR, Kraus SD (2001) Mortality and serious injury of northern right whales (Eubalaena glacialis) in the western North Atlantic Ocean. J Cetacean Res Manag 193–208
Lehnert K, Ijsseldijk LL, Uy ML, Boyi JO, van Schalkwijk L, Tollenaar EAP, Gröne A, Wohlsein P, Siebert U (2021) Whale lice (Isocyamus deltobranchium & Isocyamus delphinii; Cyamidae) prevalence in odontocetes off the German and Dutch coasts – morphological and molecular characterization and health implications. International Journal for Parasitology: Parasites and Wildlife 15:22–30
Maness HT, Nollens HH, Jensen ED, Goldstein T, LaMere S, Childress A, Sykes J, St Leger J, Lacave G, Latson FE et al (2011) Phylogenetic analysis of marine mammal herpesviruses. Vet Microbiol 149(1–2):23–29
Manire CA, Smolarek KA, Romero CH, Kinsel MJ, Clauss TM, Byrd L (2006) Proliferative dermatitis associated with a novel alphaherpesvirus in an Atlantic bottlenose dolphin (Tursiops Truncatus). J Zoo Wildl Med 37(2):174–181, 178
McGeoch DJ, Rixon FJ, Davison AJ (2006) Topics in herpesvirus genomics and evolution. Virus Res 117(1):90–104
Melero M, Crespo-Picazo JL, Rubio-Guerri C, García-Párraga D, Sánchez-Vizcaíno JM (2015) First molecular determination of herpesvirus from two mysticete species stranded in the Mediterranean Sea. BMC Vet Res 11(1):283
Melero M, García-Párraga D, Corpa JM, Ortega J, Rubio-Guerri C, Crespo JL, Rivera-Arroyo B, Sánchez-Vizcaíno JM (2014) First molecular detection and characterization of herpesvirus and poxvirus in a Pacific walrus (Odobenus rosmarus divergens). BMC Vet Res 10(1):968
Miyoshi K, Nishida S, Sone E, Tajima Y, Makara M, Yoshioka M, Nakamura M, Yamada TK, Koike H (2011) Molecular identification of novel alpha- and gammaherpesviruses from cetaceans stranded on Japanese coasts. Zoolog Sci 28(2):126–133, 128
Nemiroff L, Wimmer T, Daoust P-Y, McAlpine DF (2010) Cetacean strandings in the Canadian Maritime provinces, 1990–2008. Can Field-Nat 124(1):32–44
Noguchi K, Shimoda H, Terada Y, Shimojima M, Kohyama K, Inoshima Y, Maeda K (2013) Isolation of a novel herpesvirus from a Pacific white-sided dolphin. Adv Virol 158(3):695–699
Ogden TH, Rosenberg MS (2006) Multiple sequence alignment accuracy and phylogenetic inference. Syst Biol 55(2):314–328
Osmond MG, Kaufman GD (1998) A Heavily Parasitized Humpback Whale (Megaptera Novaeangliae). Mar Mamm Sci 14(1):146–149
Rubio-Guerri C, Melero M, Rivera-Arroyo B, Bellière EN, Crespo JL, García-Párraga D, Esperón F, Sánchez-Vizcaíno JM (2013) Simultaneous diagnosis of Cetacean morbillivirus infection in dolphins stranded in the Spanish Mediterranean Sea in 2011 using a novel Universal Probe Library (UPL) RT-PCR assay. Vet Microbiol 165(1):109–114
Sacristán C, Esperón F, Ewbank AC, Díaz-Delgado J, Ferreira-Machado E, Costa-Silva S, Sánchez-Sarmiento AM, Groch KR, Neves E, Pereira Dutra GH et al (2019) Novel herpesviruses in riverine and marine cetaceans from South America. Acta Trop 190:220–227
Sacristán C, Ewbank AC, Duarte-Benvenuto A, Sacristán I, Zamana-Ramblas R, Costa-Silva S, Lanes Ribeiro V, Bertozzi CP, Rio Del, do Valle R, Castilho PV, Colosio AC, Marcondes MCC, Lailson-Brito J, de Freitas Azevedo A, Carvalho VL, Pessi CF, Cremer M, Esperón F, Catão-Dias JL (2024) Survey of selected viral agents (herpesvirus, adenovirus and hepatitis E virus) in liver and lung samples of cetaceans, Brazil. Sci Rep 14(1):2689
Saliki JT, Cooper EJ, Rotstein DS, Caseltine SL, Pabst DA, McLellan WA, Govett P, Harms C, Smolarek KA, Romero CH (2006) A novel gammaherpesvirus associated with genital lesions in a Blainville’s beaked whale (Mesoplodon densirostris). J Wildl Dis 42(1):142–148
Seade GCC, Cerqueira VD, Sierra E, Chaves JF, Moura MAO, Montão DP, Riet-Correa G, Oliveira CA, Siciliano S, Emin-Lima R, Costa AF, Fernández A, Bezerra Júnior PS (2017) Herpesviral infection in a Guiana dolphin (Sotalia guianensis) from the northern coast of Brazil. J Vet Diagn Invest 29(6):877–879
Sierra E, Díaz-Delgado J, Arbelo M, Andrada M, Sacchini S, Fernández A (2015) Herpesvirus-associated genital lesions in a stranded striped dolphin (Stenella coeruleoalba) in the Canary Islands, Spain. J Wildl Dis 51(3):696–702, 697
Sierra E, Fernández A, Felipe-Jiménez I, Zucca D, Díaz-Delgado J, Puig-Lozano R, Câmara N, Consoli F, Díaz-Santana P, Suárez-Santana C et al (2020) Histopathological differential diagnosis of meningoencephalitis in cetaceans: morbillivirus, herpesvirus, Toxoplasma gondii, Brucella sp., and Nasitrema sp. Front Vet Sci 7:650–650
Sierra E, Fernández A, Fernández-Maldonado C, Sacchini S, Felipe-Jiménez I, Segura-Göthlin S, Colom-Rivero A, Câmara N, Puig-Lozano R, Rambaldi AM et al (2022) Molecular characterization of herpesviral encephalitis in cetaceans: correlation with histopathological and immunohistochemical findings. Animals 12(9)
Sierra E, Sánchez S, Saliki JT, Blas-Machado U, Arbelo M, Zucca D, Fernández A (2014) Retrospective study of etiologic agents associated with nonsuppurative meningoencephalitis in stranded cetaceans in the Canary Islands. J Clin Microbiol 52(7):2390
Smolarek KA, Manire CA, Ewing RY, Saliki JT, Townsend FI, Ehlers B, Romero CH (2006) Identification of novel alpha- and gammaherpesviruses from cutaneous and mucosal lesions of dolphins and whales. J Virol Methods 136(1):261–266
Soto S, González B, Willoughby K, Maley M, Olvera A, Kennedy S, Marco A, Domingo M (2012) Systemic herpesvirus and morbillivirus co-infection in a striped dolphin (Stenella coeruleoalba). J Comp Pathol 146(2):269–273
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38(7):3022–3027
Thompson JD, Plewniak F, Poch O (1999) A comprehensive comparison of multiple sequence alignment programs. Nucleic Acids Res 27(13):2682–2690
Toms CN, Stone T, Och-Adams T (2020) Visual-only assessments of skin lesions on free-ranging common bottlenose dolphins (Tursiops truncatus): reliability and utility of quantitative tools. Mar Mamm Sci 36(3):744–773
van Beurden SJ, IJsseldijk LL, Ordonez SR, Förster C, de Vrieze G, Gröne A, Verheije MH, Kik M. (2015) Identification of a novel gammaherpesvirus associated with (muco)cutaneous lesions in harbour porpoises (Phocoena phocoena). Arch Virol 160(12):3115–3120
VanDevanter DR, Warrener P, Bennett L, Schultz ER, Coulter S, Garber RL, Rose TM (1996) Detection and analysis of diverse herpesviral species by consensus primer PCR. J Clin Microbiol 34(7):1666–1671
van Elk CE, van de Bildt MW, de Jong AA, Osterhaus AD, Kuiken T (2009) Genital herpesvirus in bottlenose dolphins (Tursiops truncatus): cultivation, epidemiology and associated pathology. J Wildl Dis 45(4):895–906
Vargas-Castro I, Crespo-Picazo JL, Rivera-Arroyo B, Sánchez R, Marco-Cabedo V, Jiménez-Martínez MÁ, Fayos M, Serdio Á, García-Párraga D, Sánchez-Vizcaíno JM (2020) Alpha- and gammaherpesviruses in stranded striped dolphins (Stenella coeruleoalba) from Spain: first molecular detection of gammaherpesvirus infection in central nervous system of odontocetes. BMC Vet Res 16(1):288
Vargas-Castro I, Melero M, Crespo-Picazo JL, MdlÁ J, Sierra E, Rubio-Guerri C, Arbelo M, Fernández A, García-Párraga D, Sánchez-Vizcaíno JM (2021) Systematic determination of herpesvirus in free-ranging cetaceans stranded in the Western Mediterranean: tissue tropism and associated lesions. Viruses 13(11):2180
Vargas-Castro I, Crespo-Picazo JL, Jiménez Martínez MÁ, Marco-Cabedo V, Muñoz-Baquero M, García-Párraga D, Sánchez-Vizcaíno JM (2023) First description of a lesion in the upper digestive mucosa associated with a novel gammaherpesvirus in a striped dolphin (Stenella coeruleoalba) stranded in the Western Mediterranean Sea. BMC Vet Res 19(1):118
Acknowledgements
The authors thank Rocío Sánchez and Débora López from VISAVET for their exceptional laboratory technical assistance. The authors thank the Institut Cavanilles de Biodiversitat i Biologia Evolutiva, University of Valencia, for collaboration on necropsies. We would like to thank to Servicio de Vida Silvestre of the Conselleria de Agricultura, Desarrollo Rural, Emergencia Climática y Transición Ecológica of the Generalitat Valenciana. We also thank ZOETIS Spain for supporting veterinary diagnosis and stranding medical response.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Ignacio Vargas-Castro is the recipient of an FPU grant from the Spanish Ministry of Science, Innovation and Universities. This work was supported by a collaborative agreement involving the Fundación Oceanografic (Valencia) and the VISAVET Center of Complutense University of Madrid.
Author information
Authors and Affiliations
Contributions
IVC wrote the manuscript, and JLCP, MAJM, MMB, VMC, DGP and JMSV helped revise it. JLCP, MMB and VMC performed external examination and collected samples. IVC performed molecular analysis and performed phylogenetic study. MAJM contributed by performing the histopathology analysis. DGP and JMSV coordinated and reviewed data collection, data analysis and manuscript writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Oceanogràfic Foundation is part of the Stranding Network of the Valencian Community thanks to an agreement between the “Ciudad de las Artes y las Ciencias” and the “Conselleria de Infraestructuras, Territorio y Medio Ambiente” by which the rights of veterinary assistance are transferred to the Oceanogràfic Foundation in cases of stranded sea turtles and cetaceans. This agreement includes the collection of samples from the carcasses of stranded cetaceans. In the collection of post-mortem tissues for research purposes, the approval of the corresponding ethics committee is not required.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vargas-Castro, I., Crespo-Picazo, J.L., Jiménez Martínez, M.Á. et al. Molecular detection of herpesvirus in a skin lesion of a humpback whale (Megaptera novaeangliae) from the Western Mediterranean Sea. Eur J Wildl Res 70, 31 (2024). https://doi.org/10.1007/s10344-024-01782-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-024-01782-7