Abstract
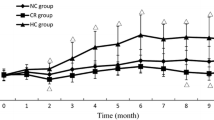
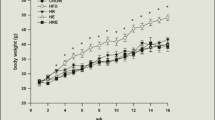
To identify the molecular mechanism underlying improved spatial learning ability of C57/BL mice on a caloric restricted (CR) diet. Seven-week-old male C57/BL mice were randomly divided into three groups: normal control group (NC group, n = 10), high energy group (n = 10), and low energy group (CR group, n = 10). Body mass and levels of blood glucose were measured every 2 weeks over the course of 30 weeks. After 30 weeks, metabolic parameters, serum total cholesterol, and insulin-like growth factor 1 (IGF-1) were measured, and learning and memory ability of animals were tested using the Morris water maze. The expression of insulin signaling pathway-related proteins in the brain tissues also were tested for molecular mechanism. When compared with the NC group, body weight, and levels of serum glucose decreased in the CR group and increased in the high energy group at all time points tested. Average escape latency and swimming distance were lower in the CR group as compared to the control group after 30 weeks. The serum cholesterol level of the high energy group was significantly higher than that of the control group. The expressions of IGF-1, IR, IRS-1, PI3K, Akt/PKB, and p-CREB protein in the CR group were significantly lower and the expressions of PI3K and Akt/PKB protein in the high energy group were significantly lower than those of the control group at post 30 weeks treatment. Our findings demonstrate that the low energy diet may improve hippocampus-dependent spatial learning ability in C57/BL mice, possibly through a regulatory mechanism of the insulin-PI3K/Akt signaling pathway.





Similar content being viewed by others
References
McCay CM, Crowell MF, Maynard LA (1935) The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr 10:63–79
Morris KC, Lin HW, Thompson JW, Perez-Pinzon MA (2011) Pathways for ischemic cytoprotection: role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J Cereb Blood Flow Metab 31:1003–1019
De Lorenzo MS, Baljinnyam E, Vatner DE, Abarzua P, Vatner SF, Rabson AB (2011) Caloric restriction reduces growth of mammary tumors and metastases. Carcinogenesis 32:1381–1387
Martin B, Mattson MP, Maudsley S (2006) Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev 5:332–353
Mladenovic Djordjevic A, Perovic M, Tesic V, Tanic N, Rakic L, Ruzdijic S, Kanazir S (2010) Long-term dietary restriction modulates the level of presynaptic proteins in the cortex and hippocampus of the aging rat. Neurochem Int 56:250–255
de la Monte SM, Wands JR (2008) Alzheimer’s disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol 2:1101–1113
Konstanze P, Juergen K, Markus S et al (2010) Insulin-resistant brain state after intracerebroventricular streptozotocin injection exacerbates Alzheimer-like changes in Tg2576 AbPP-overexpressing mice. J Alzheimers Dis 19:691–704
Guo ZH, Ersoz A, Butterfield DA, Mattson MP (2000) Beneficial effects of dietary restriction on cerebral cortical synaptic terminals: preservation of glucose and glutamate transport and mitochondrial function after exposure to amyloid beta-peptide, iron, and 3-nitropropionic acid. J Neurochem 75:314–320
Green MW, Elliman NA, Rogers PJ (1995) Lack of effect of short-term fasting on cognitive function. J Psychiatr Res 29:245–253
Argentino DP, Dominici FP, Al-Regaiey K, Bonkowski MS, Bartke A, Turyn D (2005) Effects of long-term caloric restriction on early steps of the insulin-signaling system in mouse skeletal muscle. J Gerontol A Biol Sci Med Sci 60:28–34
Fontana L, Meyer TE, Klein S et al (2004) Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. PNAS 101:6659–6663
Berryman DE, Christiansen JS, Johannsson G, Thorner MO, Kopchick JJ (2008) Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm IGF Res 18:455–471
Kenyon C (2011) The first long-lived mutants: discovery of the insulin/IGF-1 pathway for ageing. Philos Trans R Soc Lond B Biol Sci 366:9–16
Agote M, Goya L, Ramos S, Alvarez C, Gavete ML, Pascual-Leone AM, Escriva F (2001) Glucose uptake and glucose transporter proteins in skeletal muscle from undernourished rats. Am J Physiol Endocrinol Metab 281:1101–1109
Selman C, Partridge L, Withers DJ (2011) Replication of extended lifespan phenotype in mice with deletion of insulin receptor substrate. PLoS One 6:16144
Dacks PA, Moreno CL, Kim ES, Marcellino BK, Mobbs CV (2013) Role of the hypothalamus in mediating protective effects of dietary restriction during aging. Front Neuroendocrinol 34:95–106
Bergamini E, Cavallini G, Donati A, Gori Z (2003) The anti-aging effects of caloric restriction may involve stimulation of macroautophagy and lysosomal degradation, and can be intensified pharmacologically. Biomed Pharmacother 57:203–208
Lee J, Seroogy KB, Mattson MP (2002) Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem 80:539–547
Lee J, Duan W, Mattson MP (2002) Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem 82:1367–1375
Bond N, Everitt AV, Walton J (1989) Effects of dietary restriction on radial-arm maze performance and flavor memory in aged rats. Neurobiol Aging 10:27–30
Yanai S, Okaichi Y, Okaichi H (2004) Long-term dietary restriction causes negative effects on cognitive functions in rats. Neurobiol Aging 25:325–332
Acknowledgments
This work was supported by grants from the Beijing Natural Science Foundation (7132044), Capital Health Development Research Fund (2011-1001-02), Ministry of Education in China Project of Humanities and Social Sciences (12YJCZH146), and Beijing Municipal Health Bureau Research Fund (Jing 13-02).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, L., Zhao, Z., Wang, R. et al. Caloric restriction can improve learning ability in C57/BL mice via regulation of the insulin-PI3K/Akt signaling pathway. Neurol Sci 35, 1381–1386 (2014). https://doi.org/10.1007/s10072-014-1717-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-014-1717-5