Abstract
The mesopredator release hypothesis (MRH) predicts that the removal of apex predators should lead to increased abundance of smaller predators through relaxation of suppressive, top-down effects. However, apex predators’ effects on mesopredators are also likely to be modulated by interactions with human activities and ecosystem productivity. The exploitation ecosystems hypothesis (EEH) predicts that biomass of apex predators will scale with primary productivity but herbivore and mesopredator biomass will remain constant due to top-down control. Here, we take advantage of the manipulation of dingo abundance across Australia’s Dingo Fence to explore the primacy of top-down and bottom-up effects as drivers of feral cat abundance. Using field data collected across the Dingo Fence, we test the predictions generated by MRH and EEH that cat populations should be bottom-up controlled by prey abundance (a proxy for primary productivity) where top-down control exerted by dingoes was weak but not where it was strong. We examined dingo and cat scats to provide mechanistic support for the idea that dingoes control cats through killing and exploitative competition. Overall, cats were more abundant where dingoes were rare. Cat abundance was correlated positively with prey abundance where dingoes were rare but was not correlated with prey abundance where dingoes were common. Cat remains were present in 1% of dingo scats, and dietary overlap between cats and dingoes was high (0.75–0.82). Our study provides evidence that top-down control exerted by apex predators can decouple population dynamics between mesopredators and their prey and thus have primacy over bottom-up effects.
Similar content being viewed by others
Introduction
Apex predators can play a pivotal role in maintaining healthy, balanced ecosystems (Estes and others 2011). Apex predators’ suppressive, top-down, effects on the populations and phenotypes of prey and smaller predators can have cascading effects on species at lower levels in food webs and result in changes to processes that affect the flow of energy and materials through ecosystems (Ripple and others 2014; Morris and Letnic 2017; Rees and others 2017). Disruption to ecosystems caused by the removal of apex predators, such as wolves, dingoes and sharks, has been identified as a major factor contributing to the loss of biodiversity in aquatic and terrestrial systems throughout the world (Estes and others 2011; Ripple and others 2014). Consequently, restoring and maintaining the ecosystem services that top predators provide is a critical global imperative (Estes and others 2011; Ripple and others 2014).
One key pathway via which apex predators can structure ecosystems is through their suppressive effects on populations of smaller predators (Prugh and others 2009; Ritchie and Johnson 2009). Such effects can occur due to top predators directly killing smaller predators (Helldin and others 2006; Merkle and others 2009) or as a result of competitive effects that can arise if smaller predators’ ability to obtain food resources is curtailed in the presence of larger predators (Schmitz and others 2004; Swanson and others 2014; Leo and others 2015). The removal of top predators and the concomitant relaxation of suppressive top-down effects can result in a dramatic increase in abundance of smaller predators, a trophic pathway described by the mesopredator release hypothesis (MRH) (Crooks and Soulé 1999; Newsome and others 2017). However, apex predators’ effects on mesopredators do not operate in isolation but interact in complex ways with other factors such as human activities, the composition of predator and prey assemblages and the productivity of ecosystems (Greenville and others 2014; Newsome and Ripple 2015; Swanson and others 2014). Variation of apex predators’ effects on mesopredators may occur because all animals require nutrients and energy. Thus, limited availability of prey may result in bottom-up limitation when population growth is more constrained by resources than by top-down control (Letnic and Dickman 2010).
The exploitation ecosystems hypothesis (EEH) was initially invoked to explain spatial patterns in the biomasses of trophic groups (plants, herbivores and carnivores) (Rosenzweig 1971; Oksanen and others 1981). According to the EEH, variation in ecosystem productivity will cause the biomasses of each trophic group to shift to alternative equilibria in order to balance biomass exchange between them (Choquenot and Forsyth 2013). In two-link trophic systems only comprising plants and herbivores, the EEH predicts that the biomass of herbivores will increase with ecosystem productivity (Oksanen and Oksanen 2000). However, in three-link trophic systems where, in addition to plants and herbivores, apex predators are present, the EEH predicts that apex predator biomass will increase with increasing primary productivity but the biomass of herbivores will remain constant regardless of ecosystem productivity due to regulation of their populations by apex predators (Oksanen and Oksanen 2000).
Large-scale studies examining spatial trends in the abundances of predators, herbivores and primary productivity have provided strong support for the predictions of the EEH (Crête 1999; Ripple and Beschta 2012; Letnic and Crowther 2013; Letnic and Ripple 2017). Adaptations of the thinking behind the EEH have shown that it has application to explain temporal trends in plant and consumer biomass in arid ecosystems (Choquenot and Forsyth 2013; Letnic and others 2017) and also spatial trends in the abundances of apex predators, mesopredators and their prey in relation to primary productivity in a boreal context (Elmhagen and others 2010). Similar to trophic interactions in three-link plant–herbivore–predator trophic systems, we predict that the biomass of mesopredators in systems where apex predators are present should not respond to changes in the availability of prey biomass due to top-down control of mesopredator populations by apex predators. However, in the absence of apex predators’ top-down effects, mesopredator biomass should fluctuate in concert with the biomass of their prey.
The dingo (Canis dingo), the largest terrestrial carnivore in Australia, was introduced to the continent 3500–5000 years ago. Dingoes play a crucial role as top-down regulators of Australian ecosystems by regulating the abundance and in turn predatory impact of an introduced mesopredator, the red fox (Vulpes vulpes) (Johnson and VanDerWal 2009; Letnic and others 2012). The effects that dingoes have on the other introduced mesopredator in Australia, the feral cat (Felis catus), are less clear, with some studies reporting dingoes to have negative effects (Brook and others 2012; Gordon and others 2015) and some to have neutral effects (Letnic and others 2009) on feral cat abundance. One explanation for the reported variability in the numerical responses between the abundances of feral cats and dingoes is that cat populations may be influenced by interactive effects between human suppression of dingo populations, dingo abundance and prey availability (Brook and others 2012; Letnic and others 2012). Support for this idea comes from studies showing that cat populations fluctuate in response to the availability of key prey species such as European rabbits (Oryctolagus cuniculus) and small rodents whose abundances are in turn driven by rainfall-driven fluctuations in primary productivity (Read and Bowen 2001; Letnic and others 2013) and reports that dingoes’ top-down effects on cat populations were weakest during periods of high prey availability. This is because competitive effects between the coexisting predators are purported to be strongest when prey is rare and attenuated when prey availability is not a limiting resource (Greenville and others 2014).
Here, we take advantage of a long-term manipulation of the abundance of an apex predator, the dingo, in Australia’s Strzelecki Desert to explore the primacy of top-down and bottom-up effects as drivers of the abundances of feral cats (Figure 1). Populations of cats’ key prey species, small rodents and rabbits, are closely linked to rainfall-driven fluctuations in primary productivity (Read and Bowen 2001; Letnic and others . 2005; Greenville and others 2014). Applying the MRH, we predicted that cat abundance would be greater where dingo populations were rare due to persecution by humans. Applying the logic of the EEH, we predicted that top-down control exerted by dingoes would decouple the population dynamics of cats and their prey (a proxy for primary productivity). Specifically, we predicted that feral cat populations would be tightly linked to prey availability where dingoes were rare, but weakly correlated with prey abundance in environments where dingoes were abundant because increased abundance of dingoes should amplify dingoes’ top-down effects on cats. To provide mechanistic support for the idea that dingoes exert top-down effects on feral cats through direct killing and interference competition, we examined the diets of dingoes and cats on either side of Australia’s Dingo Fence to determine if dingoes killed and consumed cats and examined dietary overlap between the two predator species.
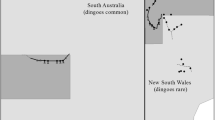
The study area in the Strzelecki Desert. Dashed lines show spotlight transects used to measure dingo, feral cat, rabbit, and dusky hopping mouse abundance indices, the thick solid line represents the Dingo Fence between South Australia (SA) and New South Wales (NSW), dark gray areas represent conservation reserves (Sturt National Park in NSW, Strzelecki Regional Reserve in SA). The inset map shows the location of the Dingo Fence (thick black line) and the location of our sample area (gray rectangle) in Australia. The flowcharts illustrate predicted differences in trophic interactions between dingoes, feral cats and their prey inside and outside the Dingo Fence. In the study areas located in South Australia, where dingoes are common, top-down control by dingoes is expected to limit cat abundance while the suppressive effects of cats on their prey should be simultaneously relaxed. In contrast, virtual absence of dingoes in the study areas located in New South Wales as a result of extensive dingo control programs should result in both an increased cat abundance and suppressive effects of cats on populations of their prey.
Methods
Study Area
We collected data at sites situated on either side of the Dingo Fence in the Strzelecki Desert, Australia, on 18 occasions at approximately four-month intervals between June 2011 and July 2017. The Dingo Fence was erected along the borders of the Australian states of New South Wales (NSW) and South Australia (SA), and NSW and Queensland (Qld) between 1914 and 1917. The fence’s purpose is to exclude dingoes from sheep grazing lands in NSW (McKnight 1969). The borders that NSW shares with SA and QLD, respectively, are arbitrary boundaries declared by royal decree prior to the region’s exploration and settlement in the 19th century along the meridians 29° S and 141° E (King William Henry the Fourth 1836). Hence, the course of the fence along the NSW border is not delineated by physical characteristics of the landscape. The fence, in combination with intensive efforts to suppress dingo populations, has reduced the population density of dingoes in western NSW to a level where the likelihood of dingo attacks on sheep (Ovis aries) is sufficiently low that sheep graziers can profitably raise sheep (McKnight 1969). Dingoes are by comparison common on the SA side of the fence where they are subject to irregular population control mainly by shooting. Kangaroos (Macropus spec.), a common prey item of dingoes (see Results section), were frequently observed inside the Dingo Fence, yet were virtually absent from survey areas located outside the fence, presumably due to extensive predation by dingoes.
We conducted surveys of the abundance of dingoes, cats, and prey species and collected scats at four study sites (two sites located east of the Dingo Fence in NSW, two sites located west of the Dingo Fence in SA; Figure 1). Sites on either side of the Dingo Fence were spaced > 50 km (NSW) and > 40 km apart (SA), respectively. Previous studies in habitat similar to our study area report maximum home ranges of dingoes to be less than 195 km2 (14.0 × 14.0 km) (Allen and others 2014) and home ranges of feral cats to not exceed 132 km2 (11.5 × 11.5 km) (Moseby and others 2009). We are thus confident that the study sites were sufficiently spaced to allow for spatial independence.
The dominant landform in the study area is parallel NE–SW trending sand dunes that can reach up to 8 m in height. Mean annual rainfall decreases from north to south and ranges from 188 to 227 mm (Australian Bureau of Meteorology). Vegetation on the sand dunes is characterized by an understory of grasses, herbs and forbs and an over-story of sparsely distributed perennial shrubs. Vegetation in the inter-dunal swales is dominated by grasses and forbs.
Abundance of Dingoes, Cats and Prey Species
Spotlight surveys are often used to measure the abundance of mammals in arid Australia because the sparse vegetation provides observers with long lines of uninterrupted sight (Letnic and Koch 2010; Gordon and others 2017; Letnic and others 2017). We indexed the abundance of dingoes, feral cats, rabbits, and dusky hopping mice (Notomys fuscus) by conducting spotlight surveys at night along narrow (< 2 m wide) unmade roads within each of the study areas. During spotlight surveys, mammals were counted by an observer who scanned a 50 W spotlight while sitting on the roof of a 4-wheel-drive vehicle (circa 2.3 m above ground level) that was moving at approximately 15 km/h. Spotlight surveys commenced at dusk and varied in length from 10 to 30 km. The total length of spotlight transects conducted at each of the four sites during each sampling session was approximately 30 km. Where multiple spotlight surveys were undertaken at a site on a survey occasion, they were always undertaken on different tracks. Because the abundance of cats was very low at sites where dingoes were common, and the abundance of dingoes, rabbits and hopping mice was very low at sites where dingoes were rare we could not use distance sampling methods to estimate densities of mammals. Consequently, for analyses, the abundance of mammals at each site was indexed as the number of individuals sighted during the survey occasion divided by the number of kilometers of spotlight survey conducted during the survey occasion.
Dingo and Cat Diet
Dingo and cat scats were collected by observers walking during the daytime along the same vehicle tracks that the spotlight surveys were conducted on. Collected scats were air-dried in paper bags before being oven-dried in the laboratory for 12 h at 100°C. After drying, individual scats were placed in nylon bags and then washed in a washing machine (Cupples and others 2011). After washing, remains in the scats were examined under a microscope and by comparing diagnostic material (that is, hair cross-sections, teeth, claws) against known reference specimens (Cupples and others 2011). The frequency of occurrence of dietary items (that is, species of prey) in the scats was calculated as the number of scats in which the dietary item was identified divided by the total number of scats sorted. A total of 109 cat scats (74 scats inside the Dingo Fence, 35 scats outside the Dingo Fence) and a total of 713 dingo scats (109 scats inside the Dingo Fence, 604 scats outside the Dingo Fence) were collected for dietary analyses.
Analyses
Abundance of Dingoes and Feral Cats in Relation to Prey Abundance
Multivariate generalized linear modeling with generalized estimating equations (GEE) (Zuur and others 2009) with a negative binomial distribution and log-link function was used to investigate the effects that prey abundance had on the abundance of feral cats and dingoes inside and outside the Dingo Fence. The location of the respective survey site inside or outside the Dingo Fence was used as a fixed factor in the analyses (“fence”). The combined indices of rabbit and hopping mouse abundance (“prey abundance”) at each site on each survey occasion were used as covariate. Because each site was subject to repeated measures, each respective sampling session was specified as a repeated measure using an AR1 error structure. All multivariable generalized linear modeling was carried out using IBM SPSS Statistics 24.0.
Dingo and Cat Diet
To determine if the respective diet of dingoes and feral cats differed between sites located inside and outside the Dingo Fence, we compared presence/absence data for each prey category in scats via the Analysis of Similarities (ANOSIM) test based on Bray–Curtis similarity matrices using the software Primer Vers. 6 (Clarke and Gorley 2006). ANOSIM is a nonparametric permutation-based approach that tests for differences between groups of multivariate samples of different treatments. ANOSIM constructs a test statistic (R) that ranges from − 1 (indicating a higher variation within than between groups) to + 1 (indicating a higher variation between than within groups).
To evaluate diet similarity for dingoes and cats on either side of the Dingo Fence, we used Pianka’s niche overlap index:
where pi is the frequency of occurrence of prey item i in the diet of species j and k (Pianka 1973). Pianka’s index (O) varies between 0 (total separation) and 1 (total overlap).
Results
Abundance of Dingoes, Feral Cats and Prey
Our spotlight surveys revealed dingoes to be virtually absent from study areas inside the Dingo Fence where we sighted on average 0.12 ± 0.08 dingoes per 100 km of transect (a total of four dingoes during our study) while dingo abundance outside the Dingo Fence was 64.5 times higher with 7.76 ± 1.55 dingoes per 100 km of transect (Wald χ2 = 36.4, p < 0.001). Cat abundance was, on average, 5.2 times higher along road transects inside the Dingo Fence (0.83 ± 0.17 cats per 100 km of transect) than outside (0.16 ± 0.05; Wald χ2 = 48.8, p < 0.001). Prey abundance was, on average, 10.7 times higher outside the Dingo Fence (514.90 ± 76.08 rabbits and hopping mice combined per 100 km of transect) than inside (48.06 ± 23.19; Wald χ2 = 22.3, p < 0.001).
Temporal Variations of Dingo, Feral Cat and Prey Abundances
Inside the Dingo Fence, cat abundance closely followed fluctuations in prey abundance during our study period (Figure 2A). Cat abundance followed a decrease in prey abundance between 2011 and 2014, increased and subsequently decreased together with prey abundance in 2014 and 2015/16, respectively, and again showed a strong increase with increasing prey abundance at the end of our study in 2017 (Figure 2A).
Abundance of dingoes, feral cats, and their prey along road transects in survey areas located A inside and B outside the Dingo Fence. Abundance is expressed as the number of individuals sighted per 100 km of spotlight transect. Prey abundance is the combined value of rabbit and hopping mouse abundance. Abundance is expressed in the number of individuals sighted per 10 km of spotlight transect.
Outside the Dingo Fence, the abundances of both cats and dingoes increased with prey abundance in 2012 and then decreased in concert with a decline in prey abundance in 2013 (Figure 2B). From 2013 onward, dingo abundance remained high and tracked trends in the abundance of their prey for the remainder of the study. However, from 2013 onward cat abundance remained low and did not scale with prey abundance (Figure 2B). Moreover, following an increase in dingo abundance with increasing prey abundance at the end of 2015, cats virtually disappeared from our study sites outside the Dingo Fence and were not recorded during in spotlight surveys conducted after November 2015 (Figure 2B).
Relationships Between Dingo, Feral Cat and Prey Abundances
With time held constant, cat abundance correlated positively with prey abundance inside the Dingo Fence. However, outside the dingo fence, the abundance of cats did not correlate with the abundance of prey (Table 1, Figure 3A). Cat abundance correlated negatively with dingo abundance outside the Dingo Fence (Table 1). Inside the Dingo Fence, the low abundance of dingoes did not allow for a statistical analysis of the correlation between dingo and cat abundance.
Dingo abundance correlated positively with prey abundance outside the Dingo Fence (Table 1, Figure 3B); inside the Dingo Fence, the low abundance of dingoes did not allow for a statistical analysis of the correlation between dingo and prey abundance.
Dingo and Cat Diet
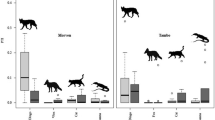
The diet of dingoes differed between study sites located inside and outside the Dingo Fence (R = 0.215, p < 0.01). The most common dietary item found in dingo scats inside the Dingo Fence was kangaroo (present in 25.7% of scats), followed by rabbit (22.9%), long-haired rat (Rattus villosissimus) (22.0%) and hopping mouse (17.4%) (Table 2). Outside the Dingo Fence, the most common dietary item was rabbit (present in 58.6% of scats), followed by long-haired rat (29.5%) and hopping mouse (22.0%) whereas remains of kangaroos were only found in 2.2% of scats (Table 2). Cat remains were found in scats collected both inside (0.9%) and outside the Dingo Fence (1.0%) (Table 2).
The diet of cats did not differ between study sites located inside and outside the Dingo Fence (R = 0.013, p = 0.27). The most common dietary item found in cat scats was rabbit (present in 35.8% of scats), followed by bird (22.0%) and hopping mouse (21.1%) (Table 2).
We found a high overlap of dingo and cat diet both inside (O = 0.75) and outside the Dingo Fence (O = 0.82), mostly attributable to the high occurrence of both rabbits and hopping mice in the diet of dingoes and cats in our study area.
Discussion
The findings of our study provide evidence that top-down effects exerted by apex predators can have a stronger influence on the abundance of mesopredators than bottom-up effects (that is, the availability of prey). In accordance with the MRH, the occurrence of cat remains in dingo scats was consistent with the idea that dingoes have the potential to exert top-down control on cat populations by directly killing them and the abundance of cats was on average higher where dingoes were rare. High dietary overlap between dingoes and cats also provides evidence that there is potential for the two species to compete for food resources. Feral cat abundance correlated with the abundance of their prey where dingoes were rare, but not where dingoes were common. These findings suggest that top-down control exerted on mesopredator populations by apex predators have the potential to decouple the population dynamics of mesopredators from their prey.
The idea that dingoes suppress populations of feral cats cat via top-down control has support from the results of our scat analysis. The occurrence of cat remains in dingo scats suggests that dingoes potentially limit feral cat populations by simply killing and thus removing individuals from the population (Moseby and others 2012). Previous studies examining dingo diets have also reported the occurrence of cat remains in dingo scats (Cupples and others 2011; Spencer and others 2014). Additionally, a study of the fate of radio-collared cats in arid Australia found that dingoes killed cats, but interestingly did not consume them (Moseby and others 2012). Because feral cats tend to occur at low population densities during the periods of dry climatic conditions that normally prevail in arid Australia (Legge and others 2017), it is conceivable that the act of a dingo killing a cat could have a marked effect on local cat populations, even if fatal encounters between dingoes and cats occur infrequently. Collectively, observations of dingoes killing cats and cat remains in dingo diets provide mechanistic support for the idea that top-down control by dingoes is the factor driving reported negative relationships between the abundances of dingoes and cats (this study; Brook and others 2012; Kennedy and others 2012; Gordon and others 2015).
Competitive interactions between predators tend to negatively affect the fitness and demography of mesopredator populations and occur when the presence of apex predators causes mesopredators to shift their dietary intake, behavior and habitat use (Creel and Creel 1996; Fedriani and others 2000). In our study, the high dietary overlap between cats and dingoes, particularly at sites where dingoes were common, suggests that considerable potential exists for dietary competition between them. However, we contend that exploitative competition between dingoes and cats at our study sites was likely to be weak because the diet of cats did not differ on either side of the dingo fence, despite great differences in prey abundance. Indeed, high abundances of rabbits and hopping mice at the sites where dingoes were common suggest that cats’ staple prey, rabbits and rodents (Doherty and others 2015), were abundantly available to both species.
A more probable mechanism for competition to occur between dingoes and cats in our study area is interference competition (Linnell and Strand 2000). To reduce the likelihood of a potentially fatal encounter with a dingo, it is conceivable that cats may increase their vigilance and modify their use of habitat. Such behavioral changes can incur significant fitness costs for mesopredators if they increase energy use, reduce consumption and possibly translate to the population level as reduced abundance (Creel and Creel 1996). We suggest that interference competition between dingoes and cats was particularly likely to occur at our study sites situated outside the Dingo Fence, because dingoes were abundant and behave agonistically toward cats (Moseby and others 2012) and because the staple dietary items of both dingoes and cats were rabbits and hopping mice. In the Strzelecki Desert, both rabbits and hopping mice focus their movements around sand dune habitats where they construct their burrows (Moseby and others 2005, 2006). This concentrated spatial distribution of prey necessitates spatial overlap in the two predators.
The positive relationship between cat and prey abundance at sites where dingoes were rare indicates that their population dynamics were closely coupled and that cat populations at those sites were subject to bottom-up control. Indeed, moderate increases in prey abundance in areas where dingoes were rare were accompanied by sharp increases in cat abundance. Presumably, these positive correlations result from higher prey abundances improving cats’ breeding success. It is possible also, that cats may aggregate at sites where prey is plentiful. The tight linkage between the abundances of cats and their prey in the virtual absence of apex predators is consistent with previous reports of positive correlations between the abundances of cats and their prey at locations on the Australian mainland where dingoes were rare (Read and Bowen 2001; Holden and Mutze 2002) and in locations in Tasmania where Tasmanian devil populations have been severely diminished by the devil facial tumor disease (Hollings and others 2014). Reports on interactions between apex predators, mesopredators and their prey in the arctic tundra corroborate these findings by demonstrating that the ability of red fox populations to respond to population declines of wolves and Eurasian lynx strongly depended on the availability of prey (Elmhagen and Rushton 2007).
Previous studies have suggested the strength of apex predators’ top-down effects on mesopredators are strongest when prey is scarce and weakest when prey is abundant because competition for prey diminishes as the abundance of prey increases (Letnic and Dickman 2010; Greenville and others 2014). Our results, which support the predictions of the EEH by showing that cat abundance increased with prey abundance where dingoes were rare but not where dingoes were common, go against the idea that top-down effects are attenuated with increasing prey availability. Instead, they suggest that dingoes’ top-down effects on cats decoupled the population dynamics of cats and their prey and suppression of cats by dingoes remained strong irrespective of increasing prey availability. The scenario that dingoes’ top-down effects on cat populations prevailed over bottom-up effects is supported also by the fact that prey was, on average, more than ten times more abundant at sites where dingoes were common than at sites where dingoes were rare. Thus, it seems unlikely that prey availability was a limiting resource for cats at sites situated outside the Dingo Fence where dingoes were common.
The EEH predicts that the magnitude of top-down effects should increase with resource availability. This is predicted to occur because increases in apex predator abundance in response to prey abundance should result in a higher rate of encounters between apex predators and lower order consumers and hence amplify top-down effects (Choquenot and Forsyth 2013). Such a situation could arise if the rate of mortality due to direct killing of cats by dingoes and competition with dingoes increased with prey availability and was equivalent to or greater than the number of cat individuals recruited into the population. Support for the idea that increasing prey availability can amplify apex predators’ top-down effects on mesopredators is provided in studies of interactions between African wild dogs, hyenas, and lions (Creel 2001). Here, high prey abundance facilitates high-density populations of lions and hyenas, which then suppress wild dog populations through direct killing and interference competition (Creel 2001).
A shortcoming of our study is that we did not experimentally manipulate dingo abundance but instead relied upon a preexisting difference in the manipulation of dingo populations across the Dingo Fence. Thus, it is possible that the differences in cat abundance observed on either side of the Dingo Fence may have been caused by other unmeasured variables. However, during the design of our study, we matched study sites for vegetation type, landform characteristics and land use. In addition, we used independent measurements of prey availability recorded at each site on each sampling occasion as covariates in the analyses. Therefore, we contend that results corroborating our hypotheses generated from the MRH and EEH would be unlikely to occur as an artifact of underlying physical or biological gradients or differences in land use. Nonetheless, it is important to note that our study provides correlative evidence only, and that further studies are required to provide experimental evidence for effects predicted to occur by the MRH and EEH (Levi and Wilmers 2012; Ford and Goheen 2015). For instance, future studies could address this limitation by experimentally reducing dingo abundance at locations outside the Dingo Fence or by reintroduction of dingoes at locations currently subject to extensive dingo control programs.
In conclusion, our study demonstrates that large fluctuations in prey availability in environments where apex predators are rare and common can have effects on the abundances of mesopredators which are analogous to those predicted by the EEH for the biomass of plants, herbivores and apex predators along spatial gradients of primary productivity. Moreover, the results of our study provide evidence that top-down control exerted by apex predators can decouple the population dynamics of mesopredators and their prey and thus have primacy over bottom-up effects. In the case of apex predator-mesopredator interactions, increased abundances of apex predators in response to prey availability may be expected to increase the rate of direct killing and intensify interference competition even though exploitation competition is simultaneously relaxed.
References
Allen BL, Engeman RM, Leung LK. 2014. The short-term effects of a routine poisoning campaign on the movements and detectability of a social top-predator. Environ Sci Pollut Res 21:2178–90.
Brook LA, Johnson CN, Ritchie EG. 2012. Effects of predator control on behaviour of an apex predator and indirect consequences for mesopredator suppression. J Appl Ecol 49:1278–86.
Choquenot D, Forsyth DM. 2013. Exploitation ecosystems and trophic cascades in non-equilibrium systems: pasture–red kangaroo–dingo interactions in arid Australia. Oikos 122:1292–306.
Clarke KR, Gorley RN. 2006. PRIMER V6: user manual-tutorial. Plymouth: Plymouth Marine Laboratory.
Creel S. 2001. Four factors modifying the effect of competition on carnivore population dynamics as illustrated by African wild dogs. Conserv Biol 15:271–4.
Creel S, Creel NM. 1996. Limitation of African wild dogs by competition with larger carnivores. Conserv Biol 10:526–38.
Crête M. 1999. The distribution of deer biomass in North America supports the hypothesis of exploitation ecosystems. Ecol Lett 2:223–7.
Crooks K, Soulé M. 1999. Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400:563–76.
Cupples JB, Crowther MS, Story G, Letnic M. 2011. Dietary overlap and prey selectivity among sympatric carnivores: could dingoes suppress foxes through competition for prey? J Mammal 92:590–600.
Doherty TS, Davis RA, van Etten EJB, Algar D, Collier N, Dickman CR, Edwards G, Masters P, Palmer R, Robinson S. 2015. A continental-scale analysis of feral cat diet in Australia. J Biogeogr 42:964–75.
Elmhagen B, Ludwig G, Rushton SP, Helle P, Lindén H. 2010. Top predators, mesopredators and their prey: interference ecosystems along bioclimatic productivity gradients. J Anim Ecol 79:785–94.
Elmhagen B, Rushton S. 2007. Trophic control of mesopredators in terrestrial ecosystems: top-down or bottom-up? Ecol Lett 10:197–206.
Estes J, Terborgh J, Brashares J. 2011. Trophic downgrading of planet Earth. Science 333:301–6.
Fedriani JM, Fuller TK, Sauvajot RM, York EC. 2000. Competition and intraguild predation among three sympatric carnivores. Oecologia 125:258–70.
Ford AT, Goheen JR. 2015. Trophic cascades by large carnivores: a case for strong inference and mechanism. Trends Ecol Evol 30:725–35.
Gordon CE, Feit A, Grüber J, Letnic M. 2015. Mesopredator suppression by an apex predator alleviates the risk of predation perceived by small prey. Proc R Soc B 282:20142870.
Gordon CE, Moore BD, Letnic M. 2017. Temporal and spatial trends in the abundances of an apex predator, introduced mesopredator and ground-nesting bird are consistent with the mesopredator release hypothesis. Biodivers Conserv 26:1445–62.
Greenville AC, Wardle GM, Tamayo B, Dickman CR. 2014. Bottom-up and top-down processes interact to modify intraguild interactions in resource-pulse environments. Oecologia 175:1349–58.
Helldin JO, Liberg O, Glöersen G. 2006. Lynx (Lynx lynx) killing red foxes (Vulpes vulpes) in boreal Sweden—frequency and population effects. J Zool 270:657–63.
Hollings T, Jones M, Mooney N, Mccallum H. 2014. Trophic cascades following the disease-induced decline of an apex predator, the Tasmanian devil. Conserv Biol 28:63–75.
Holden C, Mutze G. 2002. Impact of rabbit haemorrhagic disease on introduced predators in the Flinders Ranges, South Australia. Wildl Res 29:615–26.
Johnson CN, VanDerWal J. 2009. Evidence that dingoes limit abundance of a mesopredator in eastern Australian forests. J Appl Ecol 46:641–6.
Kennedy M, Phillips BL, Legge S, Murphy SA, Faulkner RA. 2012. Do dingoes suppress the activity of feral cats in northern Australia? Austral Ecol 37:134–9.
Legge S, Murphy BP, McGregor H, Woinarski JCZ, Augusteyn J, Ballard G, Baseler M, Buckmaster T, Dickman CR, Doherty T, Edwards G, Eyre T, Fancourt BA, Ferguson D, Forsyth DM, Geary WL, Gentle M, Gillespie G, Greenwood L, Hohnen R, Hume S, Johnson CN, Maxwell M, McDonald PJ, Morris K, Moseby K, Newsome T, Nimmo D, Paltridge R, Ramsey D, Read J, Rendall A, Rich M, Ritchie E, Rowland J, Short J, Stokeld D, Sutherland DR, Wayne AF, Woodford L, Zewe F. 2017. Enumerating a continental-scale threat: how many feral cats are in Australia? Biol Conserv 206:293–303.
Leo V, Reading RP, Letnic M. 2015. Interference competition: odours of an apex predator and conspecifics influence resource acquisition by red foxes. Oecologia 179:1033–340.
Letnic M, Crowther MS. 2013. Patterns in the abundance of kangaroo populations in arid Australia are consistent with the exploitation ecosystems hypothesis. Oikos 122:761–9.
Letnic M, Dickman CR. 2010. Resource pulses and mammalian dynamics: conceptual models for hummock grasslands and other Australian desert habitats. Biol Rev 85:501–21.
Letnic M, Feit A, Forsyth DM. 2017. Strength of a trophic cascade between an apex predator, mammalian herbivore and grasses in a desert ecosystem does not vary with temporal fluctuations in primary productivity. Ecosystems 21:153–65.
Letnic M, Koch F. 2010. Are dingoes a trophic regulator in arid Australia? A comparison of mammal communities on either side of the dingo fence. Austral Ecol 35:167–75.
Letnic M, Koch F, Gordon C, Crowther MS, Dickman CR. 2009. Keystone effects of an alien top-predator stem extinctions of native mammals. Proc R Soc B 276:3249–56.
Letnic M, Ripple WJ. 2017. Large-scale responses of herbivore prey to canid predators and primary productivity. Glob Ecol Biogeogr 26:860–6.
Letnic M, Ritchie EG, Dickman CR. 2012. Top predators as biodiversity regulators: the dingo Canis lupus dingo as a case study. Biol Rev 87:390–413.
Letnic M, Tamayo B, Dickman CR. 2005. The responses of mammals to La Niña (El Niño Southern Oscillation)—associated rainfall, predation, and wildfire in central Australia. J Mammal 86:689–703.
Letnic M, Tischler M, Gordon C. 2013. Desert small mammal responses to wildfire and predation in the aftermath of a La Niña driven resource pulse. Austral Ecol 38:841–9.
Levi T, Wilmers CC. 2012. Wolves-coyotes-foxes: a cascade among carnivores. Ecology 93:921–9.
Linnell JD, Strand O. 2000. Interference interactions, co-existence and conservation of mammalian carnivores. Divers Distributions 6:169–76.
McKnight TL. 1969. Barrier fencing for vermin control in Australia. Geogr Rev 59:330–47.
Merkle JA, Stahler DR, Smith DW. 2009. Interference competition between gray wolves and coyotes in Yellowstone National Park. Can J Zool 87:56–63.
Morris T, Letnic M. 2017. Removal of an apex predator initiates a trophic cascade that extends from herbivores to vegetation and the soil nutrient pool. Proc R Soc B 284:20170111.
Moseby KE, De Jong S, Munro N, Pieck A. 2005. Home range, activity and habitat use of European rabbits (Oryctolagus cuniculus) in arid Australia: implications for control. Wildl Res 32:305–11.
Moseby KE, Neilly H, Read JL, Crisp HA. 2012. Interactions between a top order predator and exotic mesopredators in the Australian rangelands. Int J Ecol 2012:250352.
Moseby KE, Owens H, Brandle R, Bice JK, Gates J. 2006. Variation in population dynamics and movement patterns between two geographically isolated populations of the dusky hopping mouse (Notomys fuscus). Wildl Res 33:223–32.
Moseby KE, Stott J, Crisp H. 2009. Movement patterns of feral predators in an arid environment—implications for control through poison baiting. Wildl Res 36:422–35.
Newsome TM, Greenville AC, Ćirović D, Dickman CR, Johnson CN, Krofel M, Letnic M, Ripple WJ, Ritchie EG, Stoyanov S, Wirsing AJ. 2017. Top predators constrain mesopredator distributions. Nat Commun 8:15469.
Newsome TM, Ripple WJ. 2015. A continental scale trophic cascade from wolves through coyotes to foxes. J Anim Ecol 84:49–59.
Oksanen L, Fretwell SD, Arruda J, Niemela P. 1981. Exploitation ecosystems in gradients of primary productivity. Am Nat 118:240–61.
Oksanen L, Oksanen T. 2000. The logic and realism of the hypothesis of exploitation ecosystems. Am Nat 155:703–23.
Pianka ER. 1973. The structure of lizard communities. Annu Rev Ecol Syst 4:53–74.
Prugh LR, Stoner CJ, Epps CW, Bean WT, Ripple WJ, Laliberte AS, Brashares JS. 2009. The Rise of the mesopredator. Bioscience 59:779–91.
Read J, Bowen Z. 2001. Population dynamics, diet and aspects of the biology of feral cats and foxes in arid South Australia. Wildl Res 28:195–203.
Rees JD, Kingsford RT, Letnic M. 2017. In the absence of an apex predator, irruptive herbivores suppress grass seed production: implications for small granivores. Biol Conserv 213:13–18.
Ripple WJ, Beschta RL. 2012. Large predators limit herbivore densities in northern forest ecosystems. Eur J Wildl Res 58:733–42.
Ripple WJ, Estes JA, Beschta RL, Wilmers CC, Ritchie EG, Hebblewhite M, Berger J, Elmhagen B, Letnic M, Nelson MP, Schmitz OJ, Smith DW, Wallach AD, Wirsing AJ. 2014. Status and ecological effects of the world’s largest carnivores. Science 343:1241484.
Ritchie EG, Johnson CN. 2009. Predator interactions, mesopredator release and biodiversity conservation. Ecol Lett 12:982–98.
Rosenzweig ML. 1971. Paradox of enrichment: destabilization of exploitation ecosystems in ecological time. Science 171:385–7.
Schmitz OJ, Krivan V, Ovadia O. 2004. Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol Lett 7:153–63.
Spencer EE, Crowther MS, Dickman CR. 2014. Diet and prey selectivity of three species of sympatric mammalian predators in central Australia. J Mammal 95:1278–88.
Swanson A, Caro T, Davies-Mostert H, Mills MGL, Macdonald DW, Borner M, Masenga E, Packer C. 2014. Cheetahs and wild dogs show contrasting patterns of suppression by lions. J Anim Ecol 83:1418–27.
Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. generalised estimation equations. Mixed effects models and extensions in ecology with R. New York: Springer. p 295–321.
Acknowledgements
The Australian Research Council provided funding. We thank the Ogilvy family for access to their property and support. C. Mills, C. Gordon, and N. Tong assisted with data collection. We thank the editor, two anonymous reviewers and L. Oksanen for their detailed comments and helpful suggestions during the review process, which highly improved this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
BF designed the research, collected and analyzed data and wrote the manuscript. AF collected data and wrote the manuscript. ML designed the research, collected data and wrote the manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Feit, B., Feit, A. & Letnic, M. Apex Predators Decouple Population Dynamics Between Mesopredators and Their Prey. Ecosystems 22, 1606–1617 (2019). https://doi.org/10.1007/s10021-019-00360-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-019-00360-2